Proficiency testing of diagnosis in histopathology and immunohistochemistry of breast pathology in China: results from a pilot work of National Single Disease Quality Control Program for breast cancer
- PMID: 38166768
- PMCID: PMC10763217
- DOI: 10.1186/s12885-023-11777-3
Proficiency testing of diagnosis in histopathology and immunohistochemistry of breast pathology in China: results from a pilot work of National Single Disease Quality Control Program for breast cancer
Abstract
Aim: Pathologists are currently supposed to be aware of both domestic and international guidelines for breast cancer diagnosis, but it is unclear how successfully these guidelines have been integrated into routine clinical practice in China. Thus, this national proficiency testing (PT) scheme for breast pathology was set up to conduct a baseline assessment of the diagnostic capability of pathologists in China.
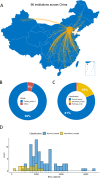
Methods: This national PT plan is designed and implemented according to the "Conformity assessment-General requirements for proficiency testing" (GB/T27043-2012/ISO/IEC 17043:2010). Five cases of breast cancer with six key items, including histologic type, grade, ER, PR, HER2, and Ki67, were selected for testing among 96 participants. The final PT results were published on the website of the National Quality Control Center for Cancer ( http://117.133.40.88:3927/cn/col22/362 ).
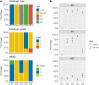
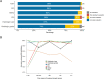
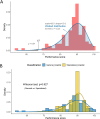
Results: Our study demonstrated that the median PT score was 89.5 (54-100). Two institutions with scores < 67 were deemed unacceptable. The accuracy of histologic type, ER, PR, HER2, and Ki67 was satisfactory (all > 86%). However, the histologic grade showed low accuracy (74.0%). The unacceptable results mainly included incorrect evaluation of histologic grade (36.7%), inaccurate evaluation of ER/PR/HER2/Ki67 (28.2%), incorrect identification of C-AD as IBC-NST (15.7%), inappropriate use of 1+/2+/3+ rather than staining percentage for ER/PR (6.1%), misclassification of ER/PR < 1% weak expression as positive staining (1.4%), and no evaluation of histologic grade in ILC, MC, and IMC (5.8%).
Conclusions: our nationwide PT program exhibited a satisfactory baseline assessment of the diagnostic capability of pathologists in China. More importantly, we identify some areas for further improvement.
Keywords: Accuracy; Breast pathology; Histopathology; Immunohistochemistry; Proficiency testing; Quality control.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Challenges and improvements in HER2 scoring and histologic evaluation: insights from a national proficiency testing scheme for breast cancer diagnosis in China.Breast Cancer Res. 2024 Sep 3;26(1):128. doi: 10.1186/s13058-024-01884-9. Breast Cancer Res. 2024. PMID: 39227982 Free PMC article.
-
External quality assessment (EQA) program for the immunohistochemical detection of ER, PR and Ki-67 in breast cancer: results of an interlaboratory reproducibility ring study in China.BMC Cancer. 2019 Oct 22;19(1):978. doi: 10.1186/s12885-019-6210-3. BMC Cancer. 2019. PMID: 31640622 Free PMC article.
-
Variability of predictive markers (hormone receptors, Her2, Ki67) and intrinsic subtypes of breast cancer in four consecutive years 2015-2018.J Cancer Res Clin Oncol. 2019 Dec;145(12):2983-2994. doi: 10.1007/s00432-019-03057-0. Epub 2019 Oct 18. J Cancer Res Clin Oncol. 2019. PMID: 31628534 Free PMC article.
-
Biomarker assessment and molecular testing for prognostication in breast cancer.Histopathology. 2016 Jan;68(1):70-85. doi: 10.1111/his.12795. Histopathology. 2016. PMID: 26768030 Review.
-
Impact of molecular subtypes classification concordance between preoperative core needle biopsy and surgical specimen on early breast cancer management: Single-institution experience and review of published literature.Eur J Surg Oncol. 2017 Apr;43(4):642-648. doi: 10.1016/j.ejso.2016.10.025. Epub 2016 Nov 17. Eur J Surg Oncol. 2017. PMID: 27889196 Review.
Cited by
-
Challenges and improvements in HER2 scoring and histologic evaluation: insights from a national proficiency testing scheme for breast cancer diagnosis in China.Breast Cancer Res. 2024 Sep 3;26(1):128. doi: 10.1186/s13058-024-01884-9. Breast Cancer Res. 2024. PMID: 39227982 Free PMC article.
-
Digital transformation of pathology - the European Society of Pathology expert opinion paper.Virchows Arch. 2025 Mar 31. doi: 10.1007/s00428-025-04090-w. Online ahead of print. Virchows Arch. 2025. PMID: 40164935
References
-
- Sloane JP, Amendoeira I, Apostolikas N, Bellocq JP, Bianchi S, Boecker W, Bussolati G, Coleman D, Connolly CE, Eusebi V, et al. Consistency achieved by 23 European pathologists from 12 countries in diagnosing breast Disease and reporting prognostic features of carcinomas. European Commission Working Group on breast Screening Pathology. Virchows Archiv: An International Journal of Pathology. 1999;434(1):3–10. doi: 10.1007/s004280050297. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

