Biobased polyester versus synthetic fiberglass casts for treating stable upper limb fractures in children: a randomized controlled trial
- PMID: 38166834
- PMCID: PMC10759437
- DOI: 10.1186/s12891-023-07138-7
Biobased polyester versus synthetic fiberglass casts for treating stable upper limb fractures in children: a randomized controlled trial
Abstract
Background: Stable upper limb fractures, such as radius, ulna, or distal humerus fractures, are common pediatric orthopedic traumas that are traditionally managed with cast immobilization. The commonly used synthetic fiberglass cast is light and water resistant but may promote skin itchiness during casting, which is a common complaint of patients. In addition, these diisocyanate-based casts have been proven to be toxic and may cause asthma. Herein, we introduce a novel biobased polyester cast to compare its clinical outcomes and patient satisfaction with conventional synthetic fiberglass casts.
Methods: From Feb 2022 to Nov 2022, we undertook a single-center prospective randomized trial involving 100 children with cast-immobilized stable upper limb fractures. These patients were randomized into either biobased polyester or synthetic fiberglass groups. All patients were regularly followed up till the cast removal which occurred approximately 3-4 weeks after immobilizing. Objective clinical findings and subjective patient questionnaire were all collected and analyzed.
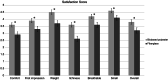
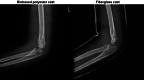
Results: According to the radiographs taken on the day of cast removal, there was no loss of reduction in both groups. The incidence of skin problems was 3.4 times higher in the synthetic fiberglass group than in the biobased polyester group. For the subjective questionnaire, the biobased polyester cast was preferred in every sub-item.
Conclusions: Our study strongly suggested that the novel biobased polyester cast provides matching stability to conventional fiberglass casts and improves patient satisfaction in an eco-friendlier and safer way.
Trial registration: ClinicalTrials.gov Protocol Registration and Results System ( https://www.
Clinicaltrials: gov/ ; ID: NCT06102603; Date: 26/10/2023).
Keywords: Biobased polyester cast; Pediatric upper limb fracture; Synthetic fiberglass cast.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Predicting initial treatment failure of fiberglass casts in pediatric distal radius fractures: utility of the second metacarpal-radius angle.J Child Orthop. 2009 Oct;3(5):375-81. doi: 10.1007/s11832-009-0198-1. Epub 2009 Aug 22. J Child Orthop. 2009. PMID: 19701659 Free PMC article.
-
Acceptability and safety of 3D printed wrist-based orthoses compared to fiberglass casts for the treatment of non-surgical distal radius- and scaphoid fractures: A randomized feasibility trial.J Hand Ther. 2025 Jan-Mar;38(1):143-151. doi: 10.1016/j.jht.2024.11.004. Epub 2025 Jan 3. J Hand Ther. 2025. PMID: 39755483 Clinical Trial.
-
Improved Comfortability and Satisfaction of Hybrid-mesh Casts in the Conservative Management of Pediatric Supracondylar Humeral Fractures: A Randomized Controlled Trial.J Pediatr Orthop. 2024 Mar 1;44(3):157-163. doi: 10.1097/BPO.0000000000002579. Epub 2023 Nov 23. J Pediatr Orthop. 2024. PMID: 37994645 Clinical Trial.
-
Waterproof casts for the management of upper limb fractures in children : a systematic review and meta-analysis.Bone Joint J. 2025 Jun 1;107-B(6):587-594. doi: 10.1302/0301-620X.107B6.BJJ-2025-0011. Bone Joint J. 2025. PMID: 40449894
-
Percutaneous pinning for treating distal radial fractures in adults.Cochrane Database Syst Rev. 2020 Feb 7;2(2):CD006080. doi: 10.1002/14651858.CD006080.pub3. Cochrane Database Syst Rev. 2020. PMID: 32032439 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

