AIM2 promotes irradiation resistance, migration ability and PD-L1 expression through STAT1/NF-κB activation in oral squamous cell carcinoma
- PMID: 38166970
- PMCID: PMC10762966
- DOI: 10.1186/s12967-023-04825-w
AIM2 promotes irradiation resistance, migration ability and PD-L1 expression through STAT1/NF-κB activation in oral squamous cell carcinoma
Abstract
Background: Radioresistance and lymph node metastasis are common phenotypes of refractory oral squamous cell carcinoma (OSCC). As a result, understanding the mechanism for radioresistance and metastatic progression is urgently needed for the precise management of refractory OSCC. Recently, immunotherapies, e.g. immune checkpoint inhibitors (ICIs), were employed to treat refractory OSCC; however, the lack of predictive biomarkers still limited their therapeutic effectiveness.
Methods: The Cancer Genome Atlas (TCGA)/Gene Expression Omnibus (GEO) databases and RT-PCR analysis were used to determine absent in melanoma 2 (AIM2) expression in OSCC samples. Colony-forming assay and trans-well cultivation was established for estimating AIM2 function in modulating the irradiation resistance and migration ability of OSCC cells, respectively. RT-PCR, Western blot and flow-cytometric analyses were performed to examine AIM2 effects on the expression of programmed death-ligand 1 (PD-L1) expression. Luciferase-based reporter assay and site-directed mutagenesis were employed to determine the transcriptional regulatory activity of Signal Transducer and Activator of Transcription 1 (STAT1) and NF-κB towards the AIM2-triggered PD-L1 expression.
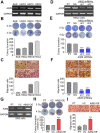
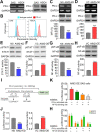
Results: Here, we found that AIM2 is extensively upregulated in primary tumors compared to the normal adjacent tissues and acts as a poor prognostic marker in OSCC. AIM2 knockdown mitigated, but overexpression promoted, radioresistance, migration and PD-L1 expression via modulating the activity of STAT1/NF-κB in OSCC cell variants. AIM2 upregulation significantly predicted a favorable response in patients receiving ICI treatments.
Conclusions: Our data unveil AIM2 as a critical factor for promoting radioresistance, metastasis and PD-L1 expression and as a potential biomarker for predicting ICI effectiveness on the refractory OSCC.
Keywords: AIM2; Immune checkpoint inhibitors; Metastasis; Oral squamous cell carcinoma; PD-L1; Radioresistance.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

