MiR-134-3p targets HMOX1 to inhibit ferroptosis in granulosa cells of sheep follicles
- PMID: 38166987
- PMCID: PMC10763389
- DOI: 10.1186/s13048-023-01328-6
MiR-134-3p targets HMOX1 to inhibit ferroptosis in granulosa cells of sheep follicles
Abstract
Background: The intricate interplay of gene expression within ovarian granulosa cells (GCs) is not fully understood. This study aimed to investigate the miRNA regulatory mechanisms of ferroptosis during the process of follicle development in lamb GCs.
Methods: Employing transcriptome sequencing, we compared differentially expressed mRNAs (DE-mRNAs) and miRNAs (DE-miRNAs) in GCs from lambs treated with follicle-stimulating hormone (FL) to untreated controls (CL). We further screened differentially expressed ferroptosis-related genes and identified potential miRNA regulatory factors. The expression patterns of HMOX1 and miRNAs in GCs were validated using qRT‒PCR and Western blotting. Additionally, we investigated the regulatory effect of oar-miR-134-3p on HMOX1 and its function in ferroptosis through cell transfection and erastin treatment.
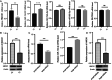
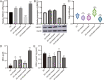
Results: We identified a total of 4,184 DE-mRNAs and 304 DE-miRNAs. The DE-mRNAs were mainly enriched in ferroptosis, insulin resistance, and the cell cycle. Specifically, we focused on the differential expression of ferroptosis-related genes. Notably, the ferroptosis-related genes HMOX1 and SLC3A2, modulated by DE-miRNAs, were markedly suppressed in FLs. Experimental validation revealed that HMOX1 was significantly downregulated in FL and large follicles, while oar-miR-134-3p was significantly upregulated compared to that in the CLs. HMOX1 expression was regulated by the targeting effect of oar-miR-134-3p. Functional assays further revealed that modulation of oar-miR-134-3p influenced HMOX1 expression and altered cellular responses to ferroptosis induction by erastin.
Conclusion: This study suggested that oar-miR-134-3p and HMOX1 may be one of the pathways regulating ferroptosis in GCs. This finding provides new clues to understanding the development and regulatory process of follicles.
Keywords: Ferroptosis; Granulosa cells; Oar-miR-134-3p; Sheep.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
Follicular fluid-derived exosomal HMOX1 promotes granulosa cell ferroptosis involved in follicular atresia in geese (Anser cygnoides).Poult Sci. 2024 Aug;103(8):103912. doi: 10.1016/j.psj.2024.103912. Epub 2024 May 29. Poult Sci. 2024. PMID: 38943808 Free PMC article.
-
Identification of differentially expressed miRNAs in serum extracellular vesicles (EVs) of Kazakh sheep at early pregnancy.Reprod Domest Anim. 2021 May;56(5):713-724. doi: 10.1111/rda.13910. Epub 2021 Mar 9. Reprod Domest Anim. 2021. PMID: 33547667
-
miRNA expression analysis of the sheep follicle during the prerecruitment, dominant, and mature stages of development under FSH stimulation.Theriogenology. 2022 Mar 15;181:161-169. doi: 10.1016/j.theriogenology.2022.01.001. Epub 2022 Jan 3. Theriogenology. 2022. PMID: 35101680
-
CircGRB14 Inhibits Proliferation and Promotes Apoptosis of Granulosa Cells in Chicken Follicle Selection Through Sponging miR-12264-3p and miR-6660-3p.Int J Mol Sci. 2025 Feb 28;26(5):2214. doi: 10.3390/ijms26052214. Int J Mol Sci. 2025. PMID: 40076832 Free PMC article.
-
Ferroptosis in AS progression: role of miRNA.Eur Rev Med Pharmacol Sci. 2022 Nov;26(22):8425-8436. doi: 10.26355/eurrev_202211_30378. Eur Rev Med Pharmacol Sci. 2022. PMID: 36459025 Review.
Cited by
-
Spermidine enhances steroidogenesis by elevating eIF5Ahyp level in hierarchical follicular granulosa cells of the goose.Poult Sci. 2025 Jul 26;104(10):105602. doi: 10.1016/j.psj.2025.105602. Online ahead of print. Poult Sci. 2025. PMID: 40749630 Free PMC article.
-
Integrated Metabolomic and Transcriptomic Analysis Reveals the Regulatory Effects of Curcumin on Bovine Ovarian Granulosa Cells.Int J Mol Sci. 2025 Jul 12;26(14):6713. doi: 10.3390/ijms26146713. Int J Mol Sci. 2025. PMID: 40724962 Free PMC article.
-
Metabolomic Analysis Identifies Betaine as a Key Mediator of TAp73α-Induced Ferroptosis in Ovarian Granulosa Cells.Int J Mol Sci. 2025 Jun 24;26(13):6045. doi: 10.3390/ijms26136045. Int J Mol Sci. 2025. PMID: 40649823 Free PMC article.
-
circAMN1-Mediated Ferroptosis Regulates the Expulsion of Placenta in Trophoblast Cells.Antioxidants (Basel). 2024 Apr 11;13(4):451. doi: 10.3390/antiox13040451. Antioxidants (Basel). 2024. PMID: 38671899 Free PMC article.
-
Quercetin mitigates iron-induced cell death in chicken granulosa cell.J Anim Sci Biotechnol. 2024 Dec 8;15(1):168. doi: 10.1186/s40104-024-01118-0. J Anim Sci Biotechnol. 2024. PMID: 39645563 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

