Midbrain node for context-specific vocalisation in fish
- PMID: 38167237
- PMCID: PMC10762186
- DOI: 10.1038/s41467-023-43794-y
Midbrain node for context-specific vocalisation in fish
Abstract
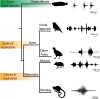
Vocalizations communicate information indicative of behavioural state across divergent social contexts. Yet, how brain regions actively pattern the acoustic features of context-specific vocal signals remains largely unexplored. The midbrain periaqueductal gray (PAG) is a major site for initiating vocalization among mammals, including primates. We show that PAG neurons in a highly vocal fish species (Porichthys notatus) are activated in distinct patterns during agonistic versus courtship calling by males, with few co-activated during a non-vocal behaviour, foraging. Pharmacological manipulations within vocally active PAG, but not hindbrain, sites evoke vocal network output to sonic muscles matching the temporal features of courtship and agonistic calls, showing that a balance of inhibitory and excitatory dynamics is likely necessary for patterning different call types. Collectively, these findings support the hypothesis that vocal species of fish and mammals share functionally comparable PAG nodes that in some species can influence the acoustic structure of social context-specific vocal signals.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
Midbrain periaqueductal gray and vocal patterning in a teleost fish.J Neurophysiol. 2006 Jul;96(1):71-85. doi: 10.1152/jn.00067.2006. Epub 2006 Apr 5. J Neurophysiol. 2006. PMID: 16598068
-
Vocal-motor and auditory connectivity of the midbrain periaqueductal gray in a teleost fish.J Comp Neurol. 2013 Mar 1;521(4):791-812. doi: 10.1002/cne.23202. J Comp Neurol. 2013. PMID: 22826153 Free PMC article.
-
Oxytocin-like receptor expression in evolutionarily conserved nodes of a vocal network associated with male courtship in a teleost fish.J Comp Neurol. 2022 Apr;530(6):903-922. doi: 10.1002/cne.25257. Epub 2021 Nov 3. J Comp Neurol. 2022. PMID: 34614539 Free PMC article.
-
The role of the periaqueductal grey in vocal behaviour.Behav Brain Res. 1994 Jun 30;62(2):107-17. doi: 10.1016/0166-4328(94)90017-5. Behav Brain Res. 1994. PMID: 7945960 Review.
-
A tale of two males: Behavioral and neural mechanisms of alternative reproductive tactics in midshipman fish.Horm Behav. 2024 May;161:105507. doi: 10.1016/j.yhbeh.2024.105507. Epub 2024 Mar 12. Horm Behav. 2024. PMID: 38479349 Review.
Cited by
-
Characterization of giant endocrine cells in the fundic stomach of African catfish (Clarias gariepinus) demonstrated by histochemical, immunohistochemical and ultrastructure microscopy methods suggesting their role in immunity.BMC Vet Res. 2024 Sep 14;20(1):415. doi: 10.1186/s12917-024-04237-y. BMC Vet Res. 2024. PMID: 39272153 Free PMC article.
References
-
- Rice AN, et al. Evolutionary patterns in sound production across fishes. Ichthyol. Herpetol. 2022;110:1–12. doi: 10.1643/i2020172. - DOI
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

