Digital Clock and Recall is superior to the Mini-Mental State Examination for the detection of mild cognitive impairment and mild dementia
- PMID: 38167251
- PMCID: PMC10759368
- DOI: 10.1186/s13195-023-01367-7
Digital Clock and Recall is superior to the Mini-Mental State Examination for the detection of mild cognitive impairment and mild dementia
Abstract
Background: Disease-modifying treatments for Alzheimer's disease highlight the need for early detection of cognitive decline. However, at present, most primary care providers do not perform routine cognitive testing, in part due to a lack of access to practical cognitive assessments, as well as time and resources to administer and interpret the tests. Brief and sensitive digital cognitive assessments, such as the Digital Clock and Recall (DCR™), have the potential to address this need. Here, we examine the advantages of DCR over the Mini-Mental State Examination (MMSE) in detecting mild cognitive impairment (MCI) and mild dementia.
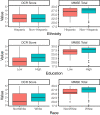
Methods: We studied 706 participants from the multisite Bio-Hermes study (age mean ± SD = 71.5 ± 6.7; 58.9% female; years of education mean ± SD = 15.4 ± 2.7; primary language English), classified as cognitively unimpaired (CU; n = 360), mild cognitive impairment (MCI; n = 234), or probable mild Alzheimer's dementia (pAD; n = 111) based on a review of medical history with selected cognitive and imaging tests. We evaluated cognitive classifications (MCI and early dementia) based on the DCR and the MMSE against cohorts based on the results of the Rey Auditory Verbal Learning Test (RAVLT), the Trail Making Test-Part B (TMT-B), and the Functional Activities Questionnaire (FAQ). We also compared the influence of demographic variables such as race (White vs. Non-White), ethnicity (Hispanic vs. Non-Hispanic), and level of education (≥ 15 years vs. < 15 years) on the DCR and MMSE scores.
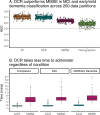
Results: The DCR was superior on average to the MMSE in classifying mild cognitive impairment and early dementia, AUC = 0.70 for the DCR vs. 0.63 for the MMSE. DCR administration was also significantly faster (completed in less than 3 min regardless of cognitive status and age). Among 104 individuals who were labeled as "cognitively unimpaired" by the MMSE (score ≥ 28) but actually had verbal memory impairment as confirmed by the RAVLT, the DCR identified 84 (80.7%) as impaired. Moreover, the DCR score was significantly less biased by ethnicity than the MMSE, with no significant difference in the DCR score between Hispanic and non-Hispanic individuals.
Conclusions: DCR outperforms the MMSE in detecting and classifying cognitive impairment-in a fraction of the time-while being not influenced by a patient's ethnicity. The results support the utility of DCR as a sensitive and efficient cognitive assessment in primary care settings.
Trial registration: ClinicalTrials.gov identifier NCT04733989.
Keywords: Alzheimer’s disease; Clock Drawing Test; Cognitive screening; Dementia; Digital cognitive assessment; Mild cognitive impairment; Mild neurocognitive disorder; Mini-Mental State Examination; Neurocognitive disorder; Rey Auditory Verbal Learning Test.
© 2023. The Author(s).
Conflict of interest statement
APL is a co-founder and Chief Medical Officer of Linus Health and a co-founder of TI Solutions and declares ownership of shares or share options in the company. APL serves as a paid member of the scientific advisory boards for Neuroelectrics, Magstim Inc., TetraNeuron, Skin2Neuron, MedRhythms, and Hearts Radiant. DB is a co-founder and Chief Executive Officer of Linus Health and declares ownership of shares or share options in the company. JS is Chief Product Officer of Linus Health and declares ownership of shares or share options in the company. All other authors are employees of Linus Health and declare ownership of shares or share options in the company.
Figures

References
-
- World Health Organization. WHO Disability and Health Fact Sheet. Cited 2023 Apr 10. Available from: https://apps.who.int/iris/rest/bitstreams/1343030/retrieve.
-
- Nichols E, Steinmetz JD, Vollset SE, Fukutaki K, Chalek J, Abd-Allah F, et al. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. Lancet Public Health. 2022;7(2):e105–e125. doi: 10.1016/S2468-2667(21)00249-8. - DOI - PMC - PubMed
-
- Gaugler J, Bryan James TJ, Reimer J, Weuve J. Alzheimer’s Disease Facts and Figures, 17. Alzheimer’s Dementia: Chicago, IL; 2021.
-
- Eisai Inc. A Placebo-Controlled, Double-Blind, Parallel-Group, 18-Month Study With an Open-Label Extension Phase to Confirm Safety and Efficacy of BAN2401 in Subjects With Early Alzheimer’s Disease. clinicaltrials.gov; 2022 . Cited 2023 May 16. Report No.: NCT03887455. Available from: https://clinicaltrials.gov/ct2/show/NCT03887455.
-
- A Study of Donanemab (LY3002813) in Participants With Early Alzheimer’s Disease (TRAILBLAZER-ALZ 2) - Full Text View - ClinicalTrials.gov. Cited 2023 May 17. Available from: https://www.clinicaltrials.gov/ct2/show/NCT04437511.
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

