Aspergillus fumigatus mitogen-activated protein kinase MpkA is involved in gliotoxin production and self-protection
- PMID: 38167253
- PMCID: PMC10762094
- DOI: 10.1038/s41467-023-44329-1
Aspergillus fumigatus mitogen-activated protein kinase MpkA is involved in gliotoxin production and self-protection
Abstract
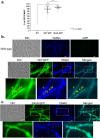
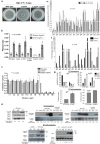
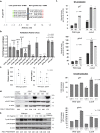
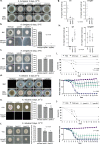
Aspergillus fumigatus is a saprophytic fungus that can cause a variety of human diseases known as aspergillosis. Mycotoxin gliotoxin (GT) production is important for its virulence and must be tightly regulated to avoid excess production and toxicity to the fungus. GT self-protection by GliT oxidoreductase and GtmA methyltransferase activities is related to the subcellular localization of these enzymes and how GT can be sequestered from the cytoplasm to avoid increased cell damage. Here, we show that GliT:GFP and GtmA:GFP are localized in the cytoplasm and in vacuoles during GT production. The Mitogen-Activated Protein kinase MpkA is essential for GT production and self-protection, interacts physically with GliT and GtmA and it is necessary for their regulation and subsequent presence in the vacuoles. The sensor histidine kinase SlnASln1 is important for modulation of MpkA phosphorylation. Our work emphasizes the importance of MpkA and compartmentalization of cellular events for GT production and self-defense.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Update of
-
Vacuoles and peroxisomes are involved in Aspergillus fumigatus gliotoxin production and self-protection.Res Sq [Preprint]. 2023 May 31:rs.3.rs-2966047. doi: 10.21203/rs.3.rs-2966047/v1. Res Sq. 2023. Update in: Nat Commun. 2024 Jan 2;15(1):33. doi: 10.1038/s41467-023-44329-1. PMID: 37398048 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

