Non-canonical interplay between glutamatergic NMDA and dopamine receptors shapes synaptogenesis
- PMID: 38167277
- PMCID: PMC10762086
- DOI: 10.1038/s41467-023-44301-z
Non-canonical interplay between glutamatergic NMDA and dopamine receptors shapes synaptogenesis
Abstract
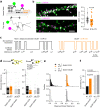
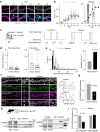
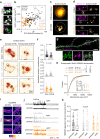
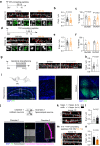
Direct interactions between receptors at the neuronal surface have long been proposed to tune signaling cascades and neuronal communication in health and disease. Yet, the lack of direct investigation methods to measure, in live neurons, the interaction between different membrane receptors at the single molecule level has raised unanswered questions on the biophysical properties and biological roles of such receptor interactome. Using a multidimensional spectral single molecule-localization microscopy (MS-SMLM) approach, we monitored the interaction between two membrane receptors, i.e. glutamatergic NMDA (NMDAR) and G protein-coupled dopamine D1 (D1R) receptors. The transient interaction was randomly observed along the dendritic tree of hippocampal neurons. It was higher early in development, promoting the formation of NMDAR-D1R complexes in an mGluR5- and CK1-dependent manner, favoring NMDAR clusters and synaptogenesis in a dopamine receptor signaling-independent manner. Preventing the interaction in the neonate, and not adult, brain alters in vivo spontaneous neuronal network activity pattern in male mice. Thus, a weak and transient interaction between NMDAR and D1R plays a structural and functional role in the developing brain.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

