Distinct forebrain regions define a dichotomous astrocytic profile in multiple system atrophy
- PMID: 38167307
- PMCID: PMC10759635
- DOI: 10.1186/s40478-023-01699-3
Distinct forebrain regions define a dichotomous astrocytic profile in multiple system atrophy
Abstract
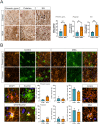
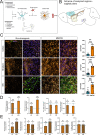
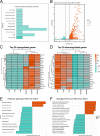
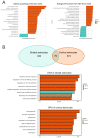
The growing recognition of a dichotomous role of astrocytes in neurodegenerative processes has heightened the need for unraveling distinct astrocytic subtypes in neurological disorders. In multiple system atrophy (MSA), a rare, rapidly progressing atypical Parkinsonian disease characterized by increased astrocyte reactivity. However the specific contribution of astrocyte subtypes to neuropathology remains elusive. Hence, we first set out to profile glial fibrillary acidic protein levels in astrocytes across the human post mortem motor cortex, putamen, and substantia nigra of MSA patients and observed an overall profound astrocytic response. Matching the post mortem human findings, a similar astrocytic phenotype was present in a transgenic MSA mouse model. Notably, MSA mice exhibited a decreased expression of the glutamate transporter 1 and glutamate aspartate transporter in the basal ganglia, but not the motor cortex. We developed an optimized astrocyte isolation protocol based on magnetic-activated cell sorting via ATPase Na+/K+ transporting subunit beta 2 and profiled the transcriptomic landscape of striatal and cortical astrocytes in transgenic MSA mice. The gene expression profile of astrocytes in the motor cortex displayed an anti-inflammatory signature with increased oligodendroglial and pro-myelinogenic expression pattern. In contrast, striatal astrocytes were defined by elevated pro-inflammatory transcripts accompanied by dysregulated genes involved in homeostatic functions for lipid and calcium metabolism. These findings provide new insights into a region-dependent, dichotomous astrocytic response-potentially beneficial in the cortex and harmful in the striatum-in MSA suggesting a differential role of astrocytes in MSA-related neurodegenerative processes.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that appeared to influence the work reported in this paper.
Figures



Similar articles
-
Degeneration in different parkinsonian syndromes relates to astrocyte type and astrocyte protein expression.J Neuropathol Exp Neurol. 2009 Oct;68(10):1073-83. doi: 10.1097/NEN.0b013e3181b66f1b. J Neuropathol Exp Neurol. 2009. PMID: 19918119
-
The stimulator of interferon genes (STING) pathway is upregulated in striatal astrocytes of patients with multiple system atrophy.Neurosci Lett. 2021 Jul 13;757:135972. doi: 10.1016/j.neulet.2021.135972. Epub 2021 May 24. Neurosci Lett. 2021. PMID: 34033888
-
Progressive striatonigral degeneration in a transgenic mouse model of multiple system atrophy: translational implications for interventional therapies.Acta Neuropathol Commun. 2018 Jan 3;6(1):2. doi: 10.1186/s40478-017-0504-y. Acta Neuropathol Commun. 2018. PMID: 29298733 Free PMC article.
-
Is Multiple System Atrophy a Prion-like Disorder?Int J Mol Sci. 2021 Sep 18;22(18):10093. doi: 10.3390/ijms221810093. Int J Mol Sci. 2021. PMID: 34576255 Free PMC article. Review.
-
Animal models of multiple system atrophy.Neuroscience. 2012 Jun 1;211:77-82. doi: 10.1016/j.neuroscience.2011.09.044. Epub 2011 Sep 25. Neuroscience. 2012. PMID: 21963351 Review.
Cited by
-
Lipids and α-Synuclein: adding further variables to the equation.Front Mol Biosci. 2024 Aug 12;11:1455817. doi: 10.3389/fmolb.2024.1455817. eCollection 2024. Front Mol Biosci. 2024. PMID: 39188788 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

