Critical contribution of mitochondria in the development of cardiomyopathy linked to desmin mutation
- PMID: 38167524
- PMCID: PMC10763022
- DOI: 10.1186/s13287-023-03619-7
Critical contribution of mitochondria in the development of cardiomyopathy linked to desmin mutation
Abstract
Background: Beyond the observed alterations in cellular structure and mitochondria, the mechanisms linking rare genetic mutations to the development of heart failure in patients affected by desmin mutations remain unclear due in part, to the lack of relevant human cardiomyocyte models.
Methods: To shed light on the role of mitochondria in these mechanisms, we investigated cardiomyocytes derived from human induced pluripotent stem cells carrying the heterozygous DESE439K mutation that were either isolated from a patient or generated by gene editing. To increase physiological relevance, cardiomyocytes were either cultured on an anisotropic micropatterned surface to obtain elongated and aligned cardiomyocytes, or as a cardiac spheroid to create a micro-tissue. Moreover, when applicable, results from cardiomyocytes were confirmed with heart biopsies of suddenly died patient of the same family harboring DESE439K mutation, and post-mortem heart samples from five control healthy donors.
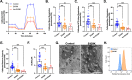
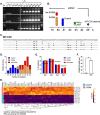
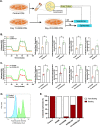
Results: The heterozygous DESE439K mutation leads to dramatic changes in the overall cytoarchitecture of cardiomyocytes, including cell size and morphology. Most importantly, mutant cardiomyocytes display altered mitochondrial architecture, mitochondrial respiratory capacity and metabolic activity reminiscent of defects observed in patient's heart tissue. Finally, to challenge the pathological mechanism, we transferred normal mitochondria inside the mutant cardiomyocytes and demonstrated that this treatment was able to restore mitochondrial and contractile functions of cardiomyocytes.
Conclusions: This work highlights the deleterious effects of DESE439K mutation, demonstrates the crucial role of mitochondrial abnormalities in the pathophysiology of desmin-related cardiomyopathy, and opens up new potential therapeutic perspectives for this disease.
Keywords: Cardiomyocytes; Desmin; Dilated cardiomyopathy; Heart failure; Mitochondria; Myofibrillar myopathy; Stem cells; iPSC.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




Similar articles
-
Integrated phenotypic and transcriptomic characterization of desmin-related cardiomyopathy in hiPSC-derived cardiomyocytes and machine learning-based classification of disease features.Eur J Cell Biol. 2025 Sep;104(3):151502. doi: 10.1016/j.ejcb.2025.151502. Epub 2025 Jul 1. Eur J Cell Biol. 2025. PMID: 40639258
-
Aberrant Mitochondrial Fission Is Maladaptive in Desmin Mutation-Induced Cardiac Proteotoxicity.J Am Heart Assoc. 2018 Jul 9;7(14):e009289. doi: 10.1161/JAHA.118.009289. J Am Heart Assoc. 2018. PMID: 29987122 Free PMC article.
-
Novel Desmin Mutation p.Glu401Asp Impairs Filament Formation, Disrupts Cell Membrane Integrity, and Causes Severe Arrhythmogenic Left Ventricular Cardiomyopathy/Dysplasia.Circulation. 2018 Apr 10;137(15):1595-1610. doi: 10.1161/CIRCULATIONAHA.117.028719. Epub 2017 Dec 6. Circulation. 2018. PMID: 29212896
-
Pathophysiological mechanisms of cardiomyopathies induced by desmin gene variants located in the C-Terminus of segment 2B.J Cell Physiol. 2024 May;239(5):e31254. doi: 10.1002/jcp.31254. Epub 2024 Mar 19. J Cell Physiol. 2024. PMID: 38501553 Review.
-
Desmin-related cardiomyopathy: an unfolding story.Am J Physiol Heart Circ Physiol. 2011 Oct;301(4):H1220-8. doi: 10.1152/ajpheart.00601.2011. Epub 2011 Jul 22. Am J Physiol Heart Circ Physiol. 2011. PMID: 21784990 Free PMC article. Review.
Cited by
-
Natural History, Phenotype Spectrum, and Clinical Outcomes of Desmin (DES)-Associated Cardiomyopathy.Circ Genom Precis Med. 2025 Apr;18(2):e004878. doi: 10.1161/CIRCGEN.124.004878. Epub 2025 Feb 19. Circ Genom Precis Med. 2025. PMID: 39968648
-
Induced Pluripotent Stem Cells in Cardiomyopathy: Advancing Disease Modeling, Therapeutic Development, and Regenerative Therapy.Int J Mol Sci. 2025 May 22;26(11):4984. doi: 10.3390/ijms26114984. Int J Mol Sci. 2025. PMID: 40507801 Free PMC article. Review.
-
Dilated cardiomyopathy: from genes and molecules to potential treatments.Mol Cell Biochem. 2025 Aug;480(8):4549-4571. doi: 10.1007/s11010-025-05269-0. Epub 2025 Mar 29. Mol Cell Biochem. 2025. PMID: 40155570 Review.
-
Amyloidogenic immunoglobulin light chains disturb contractile function and calcium transients in a human cardiac spheroid model of light chain (AL) amyloidosis.Sci Rep. 2025 Feb 4;15(1):4292. doi: 10.1038/s41598-024-82442-3. Sci Rep. 2025. PMID: 39905088 Free PMC article.
-
Natural History, Phenotype Spectrum and Clinical Outcomes of Desmin (DES)-Associated Cardiomyopathy.medRxiv [Preprint]. 2024 Aug 26:2024.08.24.24311904. doi: 10.1101/2024.08.24.24311904. medRxiv. 2024. Update in: Circ Genom Precis Med. 2025 Apr;18(2):e004878. doi: 10.1161/CIRCGEN.124.004878. PMID: 39252922 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

