Assessment of lipolysis biomarkers in adipose tissue of patients with gastrointestinal cancer
- PMID: 38167536
- PMCID: PMC10762976
- DOI: 10.1186/s40170-023-00329-9
Assessment of lipolysis biomarkers in adipose tissue of patients with gastrointestinal cancer
Abstract
Background: Adipose tissue metabolism may be impaired in patients with cancer. In particular, increased lipolysis was described in cancer-promoting adipose tissue atrophy. For this reason, we assessed the expression of the lipolysis-associated genes and proteins in subcutaneous adipose tissue (SAT) of gastrointestinal (GI) cancer patients compared to controls to verify their involvement in cancer, among different types of GI cancers, and in cachexia.
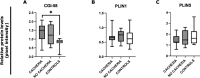
Methods: We considered patients with GI cancer (gastric, pancreatic, and colorectal) at their first diagnosis, with/without cachexia, and controls with benign diseases. We collected SAT and total RNA was extracted and ATGL, HSL, PPARα, and MCP1 were analyzed by qRT-PCR. Western blot was performed to evaluate CGI-58, PLIN1 and PLIN5.
Results: We found higher expression of ATGL and HSL in GI cancer patients with respect to controls (p ≤ 0.008) and a trend of increase for PPARα (p = 0.055). We found an upregulation of ATGL in GI cancer patients with cachexia (p = 0.033) and without cachexia (p = 0.017) vs controls. HSL was higher in patients with cachexia (p = 0.020) and without cachexia (p = 0.021), compared to controls. ATGL was upregulated in gastric cancer vs controls (p = 0.014) and higher HSL was found in gastric (p = 0.008) and in pancreatic cancer (p = 0.033) vs controls. At the protein level, we found higher CGI-58 in cancer vs controls (p = 0.019) and in cachectic vs controls (p = 0.029), as well as in gastric cancer vs controls (p = 0.027).
Conclusion: In our cohort of GI cancer patients, we found a modulation in the expression of genes and proteins involved in lipolysis, and differences were interestingly detected according to cancer type.
Keywords: Cachexia; Cancer; Gene expression, Proteins; Lipolysis.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
LinkOut - more resources
Full Text Sources
Research Materials

