A novel regulatory sex-skewing method that inhibits testicular DPY30 expression to increase female rate of dairy goat offspring
- PMID: 38167777
- PMCID: PMC10998464
- DOI: 10.1093/jas/skad422
A novel regulatory sex-skewing method that inhibits testicular DPY30 expression to increase female rate of dairy goat offspring
Abstract
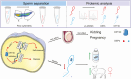
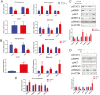
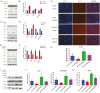
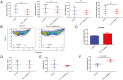
The demand for goat milk products has increased exponentially with the growth of the global population. The shortage of dairy products will be addressed extraordinarily by manipulating the female rate of goat offspring to expand the goat population and goat milk yield. No studies have reported bioinformatic analyses of X- and Y-bearing sperm of dairy goats, although this will contribute to exploring novel and applied sex-skewing technologies. Regulatory subunit of the histone methyltransferase complex (DPY30) was determined to be the key differentially expressed protein (DEP) among 15 DEPs identified in the present study. The spatiotemporal expression of DPY30 strongly suggested a functional involvement of the protein in spermatogenesis. DPY30 promoted meiosis via upregulating SYCP3, which played a crucial role in mediating sex ratio skewing in goats. Although DPY30 suppressed the self-renewal of spermatogonia stem cells through AKT/PLZF, DPY30 inhibition in the testis did not induce testicular dysgenesis. Based on the biosafety assessment in mice testes, lentivirus-mediated DPY30 knockdown in bucks' testes increased X-bearing sperm proportion and female kids' rate (22.8 percentage points) without affecting sperm quality, pregnancy rate, and kidding rate. This study provides the first evidence of the DEGs in the sexed sperm of dairy goats. DPY30 inhibition in the testes of bucks increased the female kids' rate without influencing reproductive performance. The present study provides evidence for expanding the female dairy goat population to address the concern of dairy product shortage.
Keywords: AKT; DPY30; proteomics; self-renewal; sex control.
Plain language summary
Goat milk has high digestibility, high nutritional quality, low allergenicity, and potential nutraceutical properties so the valorization of goat milk into value-added products is becoming increasingly important. However, the goat’s milk production was less than 20% of cow’s milk. To increase production, we investigated the differentially expressed proteins in the X- and Y-bearing sperm of dairy goat to explore the new sex-skewing method. The results showed that inhibiting the expression of DPY30 in the testes of male goats significantly increased the female kids’ rate (22.8 percentage points). As such, no adverse effects on sperm quality, pregnancy rate or kidding rate were observed. The DPY30 silence mediated sex-skewing was achieved by disrupting meiosis via targeting SYCP3. Our results provide new insights into the preliminary mechanisms of sex-skewing in dairy goats, which could also form the basis for the development of novel sex-skewing strategies in livestock.
© The Author(s) 2024. Published by Oxford University Press on behalf of the American Society of Animal Science. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Diluent pH affects sperm motility via GSK3 α/β-hexokinase pathway for the efficient enrichment of X-sperm to increase the female kids rate of dairy goats.Theriogenology. 2023 Apr 15;201:1-11. doi: 10.1016/j.theriogenology.2023.02.012. Epub 2023 Feb 16. Theriogenology. 2023. PMID: 36801817
-
Melatonin alleviates heat stress-induced spermatogenesis dysfunction in male dairy goats by regulating arachidonic acid metabolism mediated by remodeling the gut microbiota.Microbiome. 2024 Nov 12;12(1):233. doi: 10.1186/s40168-024-01942-6. Microbiome. 2024. PMID: 39533343 Free PMC article.
-
Alkaline semen diluent combined with R848 for separation and enrichment of dairy goat X-sperm.J Dairy Sci. 2022 Nov;105(12):10020-10032. doi: 10.3168/jds.2022-22115. Epub 2022 Oct 19. J Dairy Sci. 2022. PMID: 36270871
-
Perspectives for Buck Kids in Dairy Goat Farming.Front Vet Sci. 2021 Oct 15;8:662102. doi: 10.3389/fvets.2021.662102. eCollection 2021. Front Vet Sci. 2021. PMID: 34722689 Free PMC article. Review.
-
[Goat's milk in nutrition].Ann Pharm Fr. 2001 Feb;59(1):51-62. Ann Pharm Fr. 2001. PMID: 11223579 Review. French.
References
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

