Membrane transformations of fusion and budding
- PMID: 38167896
- PMCID: PMC10761761
- DOI: 10.1038/s41467-023-44539-7
Membrane transformations of fusion and budding
Abstract
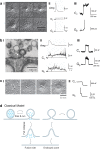
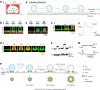
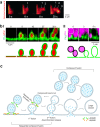
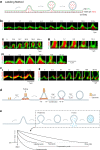
Membrane fusion and budding mediate fundamental processes like intracellular trafficking, exocytosis, and endocytosis. Fusion is thought to open a nanometer-range pore that may subsequently close or dilate irreversibly, whereas budding transforms flat membranes into vesicles. Reviewing recent breakthroughs in real-time visualization of membrane transformations well exceeding this classical view, we synthesize a new model and describe its underlying mechanistic principles and functions. Fusion involves hemi-to-full fusion, pore expansion, constriction and/or closure while fusing vesicles may shrink, enlarge, or receive another vesicle fusion; endocytosis follows exocytosis primarily by closing Ω-shaped profiles pre-formed through the flat-to-Λ-to-Ω-shape transition or formed via fusion. Calcium/SNARE-dependent fusion machinery, cytoskeleton-dependent membrane tension, osmotic pressure, calcium/dynamin-dependent fission machinery, and actin/dynamin-dependent force machinery work together to generate fusion and budding modes differing in pore status, vesicle size, speed and quantity, controls release probability, synchronization and content release rates/amounts, and underlies exo-endocytosis coupling to maintain membrane homeostasis. These transformations, underlying mechanisms, and functions may be conserved for fusion and budding in general.
© 2024. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

