Follicle stimulating hormone controls granulosa cell glutamine synthesis to regulate ovulation
- PMID: 38167949
- PMCID: PMC11214834
- DOI: 10.1093/procel/pwad065
Follicle stimulating hormone controls granulosa cell glutamine synthesis to regulate ovulation
Abstract
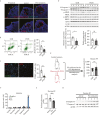
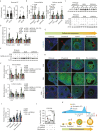
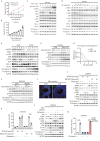
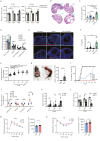
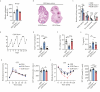
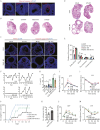
Polycystic ovary syndrome (PCOS) is the leading cause of anovulatory infertility. Inadequate understanding of the ovulation drivers hinders PCOS intervention. Herein, we report that follicle stimulating hormone (FSH) controls follicular fluid (FF) glutamine levels to determine ovulation. Murine ovulation starts from FF-exposing granulosa cell (GC) apoptosis. FF glutamine, which decreases in pre-ovulation porcine FF, elevates in PCOS patients FF. High-glutamine chow to elevate FF glutamine inhibits mouse GC apoptosis and induces hormonal, metabolic, and morphologic PCOS traits. Mechanistically, follicle-development-driving FSH promotes GC glutamine synthesis to elevate FF glutamine, which maintain follicle wall integrity by inhibiting GC apoptosis through inactivating ASK1-JNK apoptotic pathway. FSH and glutamine inhibit the rapture of cultured murine follicles. Glutamine removal or ASK1-JNK pathway activation with metformin or AT-101 reversed PCOS traits in PCOS models that are induced with either glutamine or EsR1-KO. These suggest that glutamine, FSH, and ASK1-JNK pathway are targetable to alleviate PCOS.
Keywords: FSH; PCOS; glutamine; granulosa cells; ovulation.
© The Author(s) 2024. Published by Oxford University Press on behalf of Higher Education Press.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Azziz R, Carmina E, Chen Z. et al. Polycystic ovary syndrome. Nat Rev Dis Primers 2016;2:16057. - PubMed
-
- Bae IH, Foote RH.. Utilization of glutamine for energy and protein-synthesis by cultured rabbit follicular oocytes. Exp Cell Res 1975;90:432–6. - PubMed
-
- Barbieri RL. Metformin for the treatment of polycystic ovary syndrome. Obstet Gynecol 2003;101:785–93. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

