Synovial microenvironment-influenced mast cells promote the progression of rheumatoid arthritis
- PMID: 38168103
- PMCID: PMC10761862
- DOI: 10.1038/s41467-023-44304-w
Synovial microenvironment-influenced mast cells promote the progression of rheumatoid arthritis
Abstract
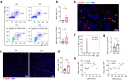
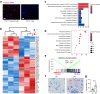
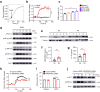
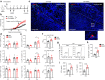
Mast cells are phenotypically and functionally heterogeneous, and their state is possibly controlled by local microenvironment. Therefore, specific analyses are needed to understand whether mast cells function as powerful participants or dispensable bystanders in specific diseases. Here, we show that degranulation of mast cells in inflammatory synovial tissues of patients with rheumatoid arthritis (RA) is induced via MAS-related G protein-coupled receptor X2 (MRGPRX2), and the expression of MHC class II and costimulatory molecules on mast cells are upregulated. Collagen-induced arthritis mice treated with a combination of anti-IL-17A and cromolyn sodium, a mast cell membrane stabilizer, show significantly reduced clinical severity and decreased bone erosion. The findings of the present study suggest that synovial microenvironment-influenced mast cells contribute to disease progression and may provide a further mast cell-targeting therapy for RA.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
All-trans-retinoic acid activated mast cells via Mas-related G-protein-coupled receptor-X2 in retinoid dermatitis.Contact Dermatitis. 2019 Sep;81(3):184-193. doi: 10.1111/cod.13292. Epub 2019 May 22. Contact Dermatitis. 2019. PMID: 31006867
-
Differential Regulation of Mas-Related G Protein-Coupled Receptor X2-Mediated Mast Cell Degranulation by Antimicrobial Host Defense Peptides and Porphyromonas gingivalis Lipopolysaccharide.Infect Immun. 2017 Sep 20;85(10):e00246-17. doi: 10.1128/IAI.00246-17. Print 2017 Oct. Infect Immun. 2017. PMID: 28694291 Free PMC article.
-
Human Mast Cell Line HMC1 Expresses Functional Mas-Related G-Protein Coupled Receptor 2.Front Immunol. 2021 Mar 15;12:625284. doi: 10.3389/fimmu.2021.625284. eCollection 2021. Front Immunol. 2021. PMID: 33790895 Free PMC article.
-
Emerging Roles for MAS-Related G Protein-Coupled Receptor-X2 in Host Defense Peptide, Opioid, and Neuropeptide-Mediated Inflammatory Reactions.Adv Immunol. 2017;136:123-162. doi: 10.1016/bs.ai.2017.06.002. Epub 2017 Jul 24. Adv Immunol. 2017. PMID: 28950944 Review.
-
Degranulation of Mast Cells as a Target for Drug Development.Cells. 2023 May 29;12(11):1506. doi: 10.3390/cells12111506. Cells. 2023. PMID: 37296626 Free PMC article. Review.
Cited by
-
P2X7 Receptor-Induced Human Mast Cell Degranulation Is Enhanced by Interleukin 33.Int J Mol Sci. 2024 Jan 31;25(3):1730. doi: 10.3390/ijms25031730. Int J Mol Sci. 2024. PMID: 38339008 Free PMC article.
-
The emerging role of vascular endothelial cell-mediated angiogenesis in the imbalance of RA synovial microenvironment and its clinical relevance.Front Pharmacol. 2025 Apr 4;16:1481089. doi: 10.3389/fphar.2025.1481089. eCollection 2025. Front Pharmacol. 2025. PMID: 40255565 Free PMC article. Review.
-
Myeloid and Mast Cell Progenitors Are Elevated in Atopic Dermatitis.JID Innov. 2025 Jun 20;5(5):100390. doi: 10.1016/j.xjidi.2025.100390. eCollection 2025 Sep. JID Innov. 2025. PMID: 40697360 Free PMC article.
-
Immune cells differentiation in osteoarthritic cartilage damage: friends or foes?Front Immunol. 2025 Mar 25;16:1545284. doi: 10.3389/fimmu.2025.1545284. eCollection 2025. Front Immunol. 2025. PMID: 40201177 Free PMC article. Review.
-
Synovial fibroblast derived small extracellular vesicles miRNA15-29148 promotes articular chondrocyte apoptosis in rheumatoid arthritis.Bone Res. 2025 Jun 12;13(1):61. doi: 10.1038/s41413-025-00430-3. Bone Res. 2025. PMID: 40506465 Free PMC article.
References
-
- Smolen JS, et al. Rheumatoid arthritis. Nat. Rev. Dis. Prim. 2018 4:1. 2018;4:1–23.
-
- Finckh A, et al. Global epidemiology of rheumatoid arthritis. Nat. Rev. Rheumatol. 2022;18:591–602. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

