This is a preprint.
AAGGG repeat expansions trigger RFC1-independent synaptic dysregulation in human CANVAS Neurons
- PMID: 38168171
- PMCID: PMC10760133
- DOI: 10.1101/2023.12.13.571345
AAGGG repeat expansions trigger RFC1-independent synaptic dysregulation in human CANVAS Neurons
Update in
-
AAGGG repeat expansions trigger RFC1-independent synaptic dysregulation in human CANVAS neurons.Sci Adv. 2024 Sep 6;10(36):eadn2321. doi: 10.1126/sciadv.adn2321. Epub 2024 Sep 4. Sci Adv. 2024. PMID: 39231235 Free PMC article.
Abstract
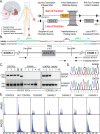
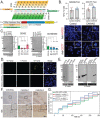
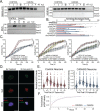
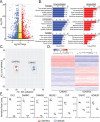
Cerebellar ataxia with neuropathy and vestibular areflexia syndrome (CANVAS) is a late onset, recessively inherited neurodegenerative disorder caused by biallelic, non-reference pentameric AAGGG(CCCTT) repeat expansions within the second intron of replication factor complex subunit 1 (RFC1). To investigate how these repeats cause disease, we generated CANVAS patient induced pluripotent stem cell (iPSC) derived neurons (iNeurons) and utilized calcium imaging and transcriptomic analysis to define repeat-elicited gain-of-function and loss-of-function contributions to neuronal toxicity. AAGGG repeat expansions do not alter neuronal RFC1 splicing, expression, or DNA repair pathway functions. In reporter assays, AAGGG repeats are translated into pentapeptide repeat proteins that selectively accumulate in CANVAS patient brains. However, neither these proteins nor repeat RNA foci were detected in iNeurons, and overexpression of these repeats in isolation did not induce neuronal toxicity. CANVAS iNeurons exhibit defects in neuronal development and diminished synaptic connectivity that is rescued by CRISPR deletion of a single expanded allele. These phenotypic deficits were not replicated by knockdown of RFC1 in control neurons and were not rescued by ectopic expression of RFC1. These findings support a repeat-dependent but RFC1-independent cause of neuronal dysfunction in CANVAS, with important implications for therapeutic development in this currently untreatable condition.
Keywords: Ataxia; C9orf72 FTD/ALS; Fragile X-associated Tremor/Ataxia Syndrome; Friedreich Ataxia; Neurodegeneration; Nucleotide Repeat expansion disorders; RAN translation; RNA foci; Short Tandem Repeats; neuropathy.
Conflict of interest statement
Conflicts of interest The authors declare no direct conflicts of interest related to the content of this manuscript. No commercial forces had editorial or supervisory input on the content of the manuscript or its figures. P.K.T. holds a shared patent on ASOs with Ionis Pharmaceuticals. He has served as a consultant with Denali Therapeutics, and he has licensed technology and antibodies to Denali and Abcam. V.K. is a co-founder of and senior advisor to DaCapo Brainscience and Yumanity Therapeutics, companies focused on CNS diseases.
Figures



References
-
- Szmulewicz David J., Roberts Leslie, McLean Catriona A., MacDougall Hamish G., Halmagyi G. Michael, and Storey Elsdon (2016). Proposed diagnostic criteria for cerebellar ataxia with neuropathy and vestibular areflexia syndrome (CANVAS). Neurology: Clinical Practice 6, 61. 10.1212/CPJ.0000000000000215. - DOI - PMC - PubMed
-
- Szmulewicz D.J., Roberts L., McLean C.A., MacDougall H.G., Chancellor A.M., Mossman S., Lamont D., Storey E., and Halmagyi G.M. (2013). Clinico-pathological correlates in cerebellar ataxia with neuronopathy and vestibular areflexia syndrome (CANVAS). Journal of the Neurological Sciences 333, e632. 10.1016/j.jns.2013.07.2196. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
