This is a preprint.
3D Mitochondrial Structure in Aging Human Skeletal Muscle: Insights into MFN-2 Mediated Changes
- PMID: 38168206
- PMCID: PMC10760012
- DOI: 10.1101/2023.11.13.566502
3D Mitochondrial Structure in Aging Human Skeletal Muscle: Insights into MFN-2 Mediated Changes
Update in
-
3D Mitochondrial Structure in Aging Human Skeletal Muscle: Insights Into MFN-2-Mediated Changes.Aging Cell. 2025 Jul;24(7):e70054. doi: 10.1111/acel.70054. Epub 2025 Apr 25. Aging Cell. 2025. PMID: 40285369 Free PMC article.
Abstract
Age-related atrophy of skeletal muscle, is characterized by loss of mass, strength, endurance, and oxidative capacity during aging. Notably, bioenergetics and protein turnover studies have shown that mitochondria mediate this decline in function. Although exercise has been the only therapy to mitigate sarcopenia, the mechanisms that govern how exercise serves to promote healthy muscle aging are unclear. Mitochondrial aging is associated with decreased mitochondrial capacity, so we sought to investigate how aging affects mitochondrial structure and potential age-related regulators. Specifically, the three-dimensional (3D) mitochondrial structure associated with morphological changes in skeletal muscle during aging requires further elucidation. We hypothesized that aging causes structural remodeling of mitochondrial 3D architecture representative of dysfunction, and this effect is mitigated by exercise. We used serial block-face scanning electron microscopy to image human skeletal tissue samples, followed by manual contour tracing using Amira software for 3D reconstruction and subsequent analysis of mitochondria. We then applied a rigorous in vitro and in vivo exercise regimen during aging. Across 5 human cohorts, we correlate differences in magnetic resonance imaging, mitochondria 3D structure, exercise parameters, and plasma immune markers between young (under 50 years) and old (over 50 years) individuals. We found that mitochondria we less spherical and more complex, indicating age-related declines in contact site capacity. Additionally, aged samples showed a larger volume phenotype in both female and male humans, indicating potential mitochondrial swelling. Concomitantly, muscle area, exercise capacity, and mitochondrial dynamic proteins showed age-related losses. Exercise stimulation restored mitofusin 2 (MFN2), one such of these mitochondrial dynamic proteins, which we show is required for the integrity of mitochondrial structure. Furthermore, we show that this pathway is evolutionarily conserved as Marf, the MFN2 ortholog in Drosophila, knockdown alters mitochondrial morphology and leads to the downregulation of genes regulating mitochondrial processes. Our results define age-related structural changes in mitochondria and further suggest that exercise may mitigate age-related structural decline through modulation of mitofusin 2.
Keywords: 3D Reconstruction; Aging; Exercise; Human Skeletal Muscle; MFN2; Mitochondria.
Conflict of interest statement
Competing Interests Disclosure All authors have no competing interests.
Figures
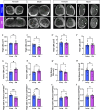



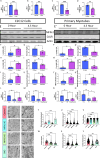

References
-
- Baar K, Wende AR, Jones TE, Marison M, Nolte LA, Chen M, Kelly DP & Holloszy JO (2002) Adaptations of skeletal muscle to exercise: rapid increase in the transcriptional coactivator PGC-1. FASEB J 16, 1879–1886. - PubMed
-
- Bartsakoulia M, Pyle A, Troncoso-Chandía D, Vial-Brizzi J, Paz-Fiblas MV, Duff J, Griffin H, Boczonadi V, Lochmüller H, Kleinle S, Chinnery PF, Grünert S, Kirschner J, Eisner V & Horvath R (2018) A novel mechanism causing imbalance of mitochondrial fusion and fission in human myopathies. Human Molecular Genetics 27, 1186–1195. - PMC - PubMed
Publication types
Grants and funding
- UL1 TR000445/TR/NCATS NIH HHS/United States
- R01 DK133698/DK/NIDDK NIH HHS/United States
- R01 HD090061/HD/NICHD NIH HHS/United States
- R21 DK119879/DK/NIDDK NIH HHS/United States
- R25 HL106365/HL/NHLBI NIH HHS/United States
- R25 HL145817/HL/NHLBI NIH HHS/United States
- T32 DK101003/DK/NIDDK NIH HHS/United States
- T32 HL007444/HL/NHLBI NIH HHS/United States
- P30 AI110527/AI/NIAID NIH HHS/United States
- UL1 RR024975/RR/NCRR NIH HHS/United States
- R00 DK120876/DK/NIDDK NIH HHS/United States
- T32 DK007563/DK/NIDDK NIH HHS/United States
- I01 BX005352/BX/BLRD VA/United States
- P30 DK020593/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
