This is a preprint.
Macrophages control pathological interferon responses during viral respiratory infection
- PMID: 38168230
- PMCID: PMC10760173
- DOI: 10.1101/2023.12.16.572019
Macrophages control pathological interferon responses during viral respiratory infection
Update in
-
Macrophage-derived oncostatin M repairs the lung epithelial barrier during inflammatory damage.Science. 2025 Jul 10;389(6756):169-175. doi: 10.1126/science.adi8828. Epub 2025 Jul 10. Science. 2025. PMID: 40638741 Free PMC article.
Abstract
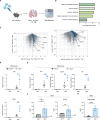
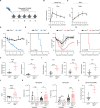
Antiviral immune mediators, including interferons and their downstream effectors, are critical for host defense yet can become detrimental when uncontrolled. Here, we identify a macrophage-mediated anti-inflammatory mechanism that limits type I interferon (IFN-I) responses. Specifically, we found that cellular stress and pathogen recognition induce Oncostatin M (OSM) production by macrophages. OSM-deficient mice succumbed to challenge with influenza or a viral mimic due to heightened IFN-I activation. Macrophage-derived OSM restricted excessive IFN-I production by lung epithelial cells following viral stimulation. Furthermore, reconstitution of OSM in the respiratory tract was sufficient to protect mice lacking macrophage-derived OSM against morbidity, indicating the importance of local OSM production. This work reveals a host strategy to dampen inflammation in the lung through the negative regulation of IFN-I by macrophages.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
