This is a preprint.
PIEZO2-dependent rapid pain system in humans and mice
- PMID: 38168273
- PMCID: PMC10760115
- DOI: 10.1101/2023.12.01.569650
PIEZO2-dependent rapid pain system in humans and mice
Abstract
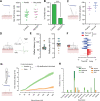
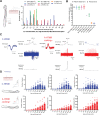
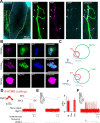
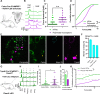
The PIEZO2 ion channel is critical for transducing light touch into neural signals but is not considered necessary for transducing acute pain in humans. Here, we discovered an exception - a form of mechanical pain evoked by hair pulling. Based on observations in a rare group of individuals with PIEZO2 deficiency syndrome, we demonstrated that hair-pull pain is dependent on PIEZO2 transduction. Studies in control participants showed that hair-pull pain triggered a distinct nocifensive response, including a nociceptive reflex. Observations in rare Aβ deafferented individuals and nerve conduction block studies in control participants revealed that hair-pull pain perception is dependent on Aβ input. Single-unit axonal recordings revealed that a class of cooling-responsive myelinated nociceptors in human skin is selectively tuned to painful hair-pull stimuli. Further, we pharmacologically mapped these nociceptors to a specific transcriptomic class. Finally, using functional imaging in mice, we demonstrated that in a homologous nociceptor, Piezo2 is necessary for high-sensitivity, robust activation by hair-pull stimuli. Together, we have demonstrated that hair-pulling evokes a distinct type of pain with conserved behavioral, neural, and molecular features across humans and mice.
Conflict of interest statement
Conflict of interest. None
Figures
References
-
- Wall P.D., McMahon S.B., and Koltzenburg M. (2006). Wall and Melzack’s textbook of pain, 5th Edition (Elsevier/Churchill Livingstone; ).
-
- Yu H., Usoskin D., Nagi S.S., Hu Y., Kupari J., Bouchatta O., Cranfill S.L., Gautam M., Su Y., Lu Y., et al. (2023). Single-Soma Deep RNA Sequencing of Human Dorsal Root Ganglion Neurons Reveals Novel Molecular and Cellular Mechanisms Underlying Somatosensation. bioRxiv, 2023.2003.2017.533207. 10.1101/2023.03.17.533207. - DOI
-
- Tavares-Ferreira D., Shiers S., Ray P.R., Wangzhou A., Jeevakumar V., Sankaranarayanan I., Cervantes A.M., Reese J.C., Chamessian A., Copits B.A., et al. (2022). Spatial transcriptomics of dorsal root ganglia identifies molecular signatures of human nociceptors. Sci Transl Med 14, eabj8186. 10.1126/scitranslmed.abj8186. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
