This is a preprint.
Genetic implication of prenatal GABAergic and cholinergic neuron development in susceptibility to schizophrenia
- PMID: 38168283
- PMCID: PMC10760267
- DOI: 10.1101/2023.12.14.23299948
Genetic implication of prenatal GABAergic and cholinergic neuron development in susceptibility to schizophrenia
Update in
-
Genetic Implication of Prenatal GABAergic and Cholinergic Neuron Development in Susceptibility to Schizophrenia.Schizophr Bull. 2024 Aug 27;50(5):1171-1184. doi: 10.1093/schbul/sbae083. Schizophr Bull. 2024. PMID: 38869145 Free PMC article.
Abstract
Background: The ganglionic eminences are fetal-specific structures that give rise to gamma-aminobutyric acid (GABA)- and acetylcholine- releasing neurons of the forebrain. Given evidence for GABAergic and cholinergic disturbances in schizophrenia, as well as an early neurodevelopmental component to the disorder, we tested the potential involvement of developing cells of the ganglionic eminences in mediating genetic risk for the condition.
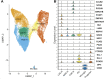
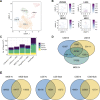
Study design: We combined data from a recent large-scale genome-wide association study of schizophrenia with single cell RNA sequencing data from the human ganglionic eminences to test enrichment of schizophrenia risk variation in genes with high expression specificity for particular developing cell populations within these structures. We additionally performed the single nuclei Assay for Transposase-Accessible Chromatin with Sequencing (snATAC-Seq) to map potential regulatory genomic regions operating in individual cell populations of the human ganglionic eminences, using these to additionally test for enrichment of schizophrenia common genetic variant liability and to functionally annotate non-coding variants associated with the disorder.
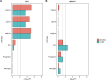
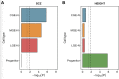
Study results: Schizophrenia common variant liability was enriched in genes with high expression specificity for developing neuron populations that are predicted to form dopamine D1 and D2 receptor expressing GABAergic medium spiny neurons of the striatum, cortical somatostatin-positive GABAergic interneurons, calretinin-positive GABAergic neurons and cholinergic neurons. Consistent with these findings, schizophrenia genetic risk was also concentrated in predicted regulatory genomic sequence mapped in developing neuronal populations of the ganglionic eminences.
Conclusions: Our study provides evidence for a role of prenatal GABAergic and cholinergic neuron development in later susceptibility to schizophrenia.
Conflict of interest statement
Conflicts of interest The Authors have declared that there are no conflicts of interest in relation to the subject of this study.
Figures

References
-
- Olsson M, Campbell K, Wictorin K, Björklund A. Projection neurons in fetal striatal transplants are predominantly derived from the lateral ganglionic eminence. Neuroscience 1995;69:1169–1182. - PubMed
-
- Nery S, Fishell G, Corbin JG. The caudal ganglionic eminence is a source of distinct cortical and subcortical cell populations. Nat Neurosci 2002;5:1279–1287. - PubMed
-
- Allaway KC, Machold R. Developmental specification of forebrain cholinergic neurons. Dev Biol 2017;421:1–7. - PubMed
-
- Akbarian S, Kim JJ, Potkin SG, et al. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch Gen Psychiatry 1995;52:258–266. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
