This is a preprint.
Keratinocyte Piezo1 drives paclitaxel-induced mechanical hypersensitivity
- PMID: 38168305
- PMCID: PMC10760029
- DOI: 10.1101/2023.12.12.571332
Keratinocyte Piezo1 drives paclitaxel-induced mechanical hypersensitivity
Update in
-
Increased keratinocyte activity and PIEZO1 signaling contribute to paclitaxel-induced mechanical hypersensitivity.Sci Transl Med. 2024 Dec 11;16(777):eadn5629. doi: 10.1126/scitranslmed.adn5629. Epub 2024 Dec 11. Sci Transl Med. 2024. PMID: 39661703
Abstract
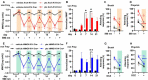
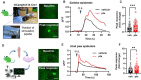
Recent work demonstrates that epidermal keratinocytes are critical for normal touch sensation. However, it is unknown if keratinocytes contribute to touch evoked pain and hypersensitivity following tissue injury. Here, we used inhibitory optogenetic and chemogenetic techniques to determine the extent to which keratinocyte activity contributes to the severe neuropathic pain that accompanies chemotherapeutic treatment. We found that keratinocyte inhibition largely alleviates paclitaxel-induced mechanical hypersensitivity. Furthermore, we found that paclitaxel exposure sensitizes mouse and human keratinocytes to mechanical stimulation through the keratinocyte mechanotransducer Piezo1. These findings demonstrate the contribution of non-neuronal cutaneous cells to neuropathic pain and pave the way for the development of new pain-relief strategies that target epidermal keratinocytes and Piezo1.
Figures




References
-
- Marupudi N. I. et al. Paclitaxel: a review of adverse toxicities and novel delivery strategies. Expert Opin. Drug Saf. 6, 609–621 (2007). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
