This is a preprint.
Improved bladder cancer antitumor efficacy with a recombinant BCG that releases a STING agonist
- PMID: 38168333
- PMCID: PMC10760079
- DOI: 10.1101/2023.12.15.571740
Improved bladder cancer antitumor efficacy with a recombinant BCG that releases a STING agonist
Abstract
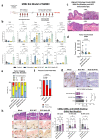
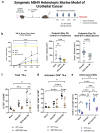
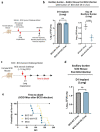
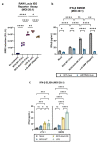
Despite the introduction of several new agents for the treatment of bladder cancer (BC), intravesical BCG remains a first line agent for the management of non-muscle invasive bladder cancer. In this study we evaluated the antitumor efficacy in animal models of BC of a recombinant BCG known as BCG-disA-OE that releases the small molecule STING agonist c-di-AMP. We found that compared to wild-type BCG (BCG-WT), in both the orthotopic, carcinogen-induced rat MNU model and the heterotopic syngeneic mouse MB-49 model BCG-disA-OE afforded improved antitumor efficacy. A mouse safety evaluation further revealed that BCG-disA-OE proliferated to lesser degree than BCG-WT in BALB/c mice and displayed reduced lethality in SCID mice. To probe the mechanisms that may underlie these effects, we found that BCG-disA-OE was more potent than BCG-WT in eliciting IFN-β release by exposed macrophages, in reprogramming myeloid cell subsets towards an M1-like proinflammatory phenotypes, inducing epigenetic activation marks in proinflammatory cytokine promoters, and in shifting monocyte metabolomic profiles towards glycolysis. Many of the parameters elevated in cells exposed to BCG-disA-OE are associated with BCG-mediated trained innate immunity suggesting that STING agonist overexpression may enhance trained immunity. These results indicate that modifying BCG to release high levels of proinflammatory PAMP molecules such as the STING agonist c-di-AMP can enhance antitumor efficacy in bladder cancer.
Conflict of interest statement
COMPETING INTERESTS M.P., W.R.B., and T.J.B. are co-inventors on patent applications involving BCG-disA-OE. W.R.B. and T.J.B. are co-founders of OncoSTING, LLC, which holds rights to commercialize BCC-disA-OE. The remaining authors declare no competing interests.
Figures



References
-
- Morales A., Eidinger D. & Bruce A.W. Intracavitary Bacillus Calmette-Guerin in the treatment of superficial bladder tumors. J Urol 116, 180–183 (1976). - PubMed
-
- Fankhauser C.D., Teoh J.Y. & Mostafid H. Treatment options and results of adjuvant treatment in nonmuscle-invasive bladder cancer (NMIBC) during the Bacillus Calmette-Guérin shortage. Curr Opin Urol 30, 365–369 (2020). - PubMed
-
- Roumiguié M., et al. International Bladder Cancer Group Consensus Statement on Clinical Trial Design for Patients with Bacillus Calmette-Guérin-exposed High-risk Non-muscle-invasive Bladder Cancer. Eur Urol 82, 34–46 (2022). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
