This is a preprint.
Role of Ribeye PXDLS/T-binding cleft in normal synaptic ribbon function
- PMID: 38168344
- PMCID: PMC10760060
- DOI: 10.1101/2023.12.12.571266
Role of Ribeye PXDLS/T-binding cleft in normal synaptic ribbon function
Abstract
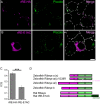
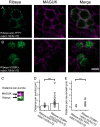
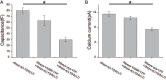
Non-spiking sensory hair cells of the auditory and vestibular systems encode a dynamic range of graded signals with high fidelity by vesicle exocytosis at ribbon synapses. Ribeye, the most abundant protein in the synaptic ribbon, is composed of a unique A domain specific for ribbons and a B-domain nearly identical to the transcriptional corepressor CtBP2. CTBP2 and the B-domain of Ribeye contain a surface cleft that binds to proteins harboring a PXDLS/T peptide motif. Little is known about the importance of this binding site in synaptic function. Piccolo has a well-conserved PVDLT motif and we find that overexpressed Ribeye exhibits striking co-localization with Piccolo in INS-cells, while two separate mutants containing mutations in PXDLS/T-binding region, fail to co-localize with Piccolo. Similarly, co-transfected Ribeye and a piccolo fragment containing the PVDLT region co-localize in HEK cells. Expression of wild-type Ribeye-YFP in zebrafish neuromast hair cells returns electron densities to ribbon structures and mostly rescued normal synaptic transmission and morphological phenotypes in a mutant zebrafish lacking most Ribeye. By contrast, Ribeye-YFP harboring a mutation in the PXDLS/T-binding cleft resulted in ectopic electron dense aggregates that did not collect vesicles and the persistence of ribbons lacking electron densities. Furthermore, overexpression failed to return capacitance responses to normal levels. These results point toward a role for the PXDLS/T-binding cleft in the recruitment of Ribeye to ribbons and in normal synaptic function.
Conflict of interest statement
The authors declare no competing financial interests.
Figures







Similar articles
-
Characterization of Ribeye subunits in zebrafish hair cells reveals that exogenous Ribeye B-domain and CtBP1 localize to the basal ends of synaptic ribbons.PLoS One. 2014 Sep 10;9(9):e107256. doi: 10.1371/journal.pone.0107256. eCollection 2014. PLoS One. 2014. PMID: 25208216 Free PMC article.
-
Synaptic Ribbons Require Ribeye for Electron Density, Proper Synaptic Localization, and Recruitment of Calcium Channels.Cell Rep. 2016 Jun 21;15(12):2784-95. doi: 10.1016/j.celrep.2016.05.045. Epub 2016 Jun 9. Cell Rep. 2016. PMID: 27292637 Free PMC article.
-
A Multiple Piccolino-RIBEYE Interaction Supports Plate-Shaped Synaptic Ribbons in Retinal Neurons.J Neurosci. 2019 Apr 3;39(14):2606-2619. doi: 10.1523/JNEUROSCI.2038-18.2019. Epub 2019 Jan 29. J Neurosci. 2019. PMID: 30696732 Free PMC article.
-
The making of synaptic ribbons: how they are built and what they do.Neuroscientist. 2009 Dec;15(6):611-24. doi: 10.1177/1073858409340253. Neuroscientist. 2009. PMID: 19700740 Review.
-
Dual use of the transcriptional repressor (CtBP2)/ribbon synapse (RIBEYE) gene: how prevalent are multifunctional genes?Trends Neurosci. 2001 Oct;24(10):555-7. doi: 10.1016/s0166-2236(00)01894-4. Trends Neurosci. 2001. PMID: 11576649 Review.
References
-
- Brandstatter JH, Fletcher EL, Garner CC, Gundelfinger ED, Wassle H (1999) Differential expression of the presynaptic cytomatrix protein bassoon among ribbon synapses in the mammalian retina. The European journal of neuroscience 11:3683–3693. - PubMed
-
- Chinnadurai G (2002) CtBP, an unconventional transcriptional corepressor in development and oncogenesis. Mol Cell 9:213–224. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
