This is a preprint.
Methamphetamine enhancement of HIV-1 gp120-mediated NLRP3 inflammasome activation and resultant proinflammatory responses in rat microglial cultures
- PMID: 38168345
- PMCID: PMC10760309
- DOI: 10.21203/rs.3.rs-3707515/v1
Methamphetamine enhancement of HIV-1 gp120-mediated NLRP3 inflammasome activation and resultant proinflammatory responses in rat microglial cultures
Update in
-
Methamphetamine Enhancement of HIV-1 gp120-Mediated NLRP3 Inflammasome Activation and Resultant Proinflammatory Responses in Rat Microglial Cultures.Int J Mol Sci. 2024 Mar 22;25(7):3588. doi: 10.3390/ijms25073588. Int J Mol Sci. 2024. PMID: 38612400 Free PMC article.
Abstract
Background: Human Immunodeficiency Virus type 1 (HIV-1)-associated neurocognitive disorders (HAND) remain prevalent in HIV-1-infected individuals despite the evident success of combined antiretroviral therapy (cART). The mechanisms under HAND prevalence in the cART era remain perplexing. Ample evidence indicates that HIV-1 envelope glycoprotein protein 120 (gp120), a potent neurotoxin, plays a pivotal role in the HAND pathogenesis. Methamphetamine (Meth) abuse exacerbates HAND. How Meth exacerbates HAND is not fully understood. This study was to test the hypothesis that Meth exacerbates HAND by enhancing gp120-mediated proinflammatory responses in the brain, worsening the pathogenesis of HAND.
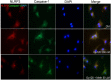
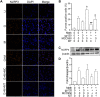
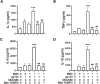
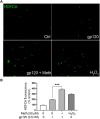
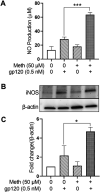
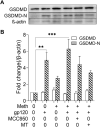
Methods: Experiments were carried out on primary microglial cultures prepared from neonatal SD rats. The purity of microglia was determined by staining with anti-CD11b. Meth and gp120 were applied to microglial cultures. Microglial activation was revealed by immunostaining and Iba-1 expression. The protein expression levels of Pro-IL-1β, Il-1β, Iba-1, iNOS, NLRP3, GSDMD and GSDMD-N were detected by western blotting analyses. The levels of proinflammatory cytokine and NO production in the microglia culture supernatants were assayed by ELISA and Griess reagent systems, respectively. NLRP3 activation was uncovered by fluorescent microscopy images displaying NLRP3 puncta labeled by anti-NLRP3 antibody. NLRP3 co-localization with caspase-1 was labeled with antibodies. One-way ANOVA with post hoc Tukey's multiple comparison tests was employed for statistical analyses.
Results: Meth enhanced gp120-induced microglia activation revealed by immunostaining and Iba-1 expression, and potentiated gp120-mediated NLRP3 expression, IL-1β processing and release assayed by immunoblot and ELISA. Meth also augmented the co-localization of NLRP3 and caspase-1, increased the numbers of NLRP3 puncta and ROS production, elevated levels of iNOS expression and NO production, and enhanced levels of cleaved gasderminD (GSDMD-N, an executor of pyroptosis) in gp120-primed microglia. The Meth-associated effects were attenuated or blocked by MCC950, an NLRP3 inhibitor, or Mito-TEMPO, a mitochondrial superoxide scavenger, indicating the involvement of mitochondria in Meth enhancement of NLRP3 inflammasome activation in gp120-primed microglia.
Conclusions: These results suggest that Meth enhanced gp120-associated microglial NLRP3 activation and resultant proinflammatory responses via mitochondria-dependent signaling.
Keywords: HIV-1gp120; Methamphetamine; NLRP3 inflammasome; microglia; neuroinflammation.
Conflict of interest statement
Competing interests The authors declare that there no competing interests.
Figures


Similar articles
-
Methamphetamine Enhancement of HIV-1 gp120-Mediated NLRP3 Inflammasome Activation and Resultant Proinflammatory Responses in Rat Microglial Cultures.Int J Mol Sci. 2024 Mar 22;25(7):3588. doi: 10.3390/ijms25073588. Int J Mol Sci. 2024. PMID: 38612400 Free PMC article.
-
Methamphetamine potentiates HIV-1gp120-induced microglial neurotoxic activity by enhancing microglial outward K+ current.Mol Cell Neurosci. 2017 Jul;82:167-175. doi: 10.1016/j.mcn.2017.05.009. Epub 2017 May 25. Mol Cell Neurosci. 2017. PMID: 28552341 Free PMC article.
-
Methamphetamine augments HIV-1 gp120 inhibition of synaptic transmission and plasticity in rat hippocampal slices: Implications for methamphetamine exacerbation of HIV-associated neurocognitive disorders.Neurobiol Dis. 2022 Jun 15;168:105712. doi: 10.1016/j.nbd.2022.105712. Epub 2022 Mar 22. Neurobiol Dis. 2022. PMID: 35337950 Free PMC article.
-
Role of microglia in methamphetamine-induced neurotoxicity.Int J Physiol Pathophysiol Pharmacol. 2017 Jun 15;9(3):84-100. eCollection 2017. Int J Physiol Pathophysiol Pharmacol. 2017. PMID: 28694920 Free PMC article. Review.
-
P2Y13 receptor involved in HIV-1 gp120 induced neuropathy in superior cervical ganglia through NLRP3 inflammasome activation.Neuropharmacology. 2024 Mar 1;245:109818. doi: 10.1016/j.neuropharm.2023.109818. Epub 2023 Dec 22. Neuropharmacology. 2024. PMID: 38142931 Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

