This is a preprint.
Loss of Endothelial TDP-43 Leads to Blood Brain Barrier Defects in Mouse Models of Amyotrophic Lateral Sclerosis and Frontotemporal Dementia
- PMID: 38168388
- PMCID: PMC10760101
- DOI: 10.1101/2023.12.13.571184
Loss of Endothelial TDP-43 Leads to Blood Brain Barrier Defects in Mouse Models of Amyotrophic Lateral Sclerosis and Frontotemporal Dementia
Update in
-
Amyotrophic lateral sclerosis and frontotemporal dementia mutation reduces endothelial TDP-43 and causes blood-brain barrier defects.Sci Adv. 2025 Apr 18;11(16):eads0505. doi: 10.1126/sciadv.ads0505. Epub 2025 Apr 16. Sci Adv. 2025. PMID: 40238886 Free PMC article.
Abstract
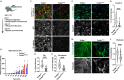
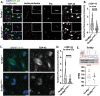
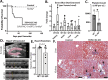
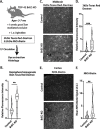
Loss of nuclear TDP-43 occurs in a wide range of neurodegenerative diseases, and specific mutations in the TARDBP gene that encodes the protein are linked to familial Frontal Temporal Lobar Dementia (FTD), and Amyotrophic Lateral Sclerosis (ALS). Although the focus has been on neuronal cell dysfunction caused by TDP-43 variants, TARDBP mRNA transcripts are expressed at similar levels in brain endothelial cells (ECs). Since increased permeability across the blood brain barrier (BBB) precedes cognitive decline, we postulated that altered functions of TDP-43 in ECs contributes to BBB dysfunction in neurodegenerative disease. To test this hypothesis, we examined EC function and BBB properties in mice with either knock-in mutations found in ALS/FTLD patients (TARDBP G348C and GRN R493X ) or EC-specific deletion of TDP-43 throughout the endothelium (Cdh5(PAC)CreERT2; Tardbp ff ) or restricted to brain endothelium (Slco1c1(BAC)CreERT2; Tardbp ff ). We found that TARDBP G348C mice exhibited increased permeability to 3kDa Texas Red dextran and NHS-biotin, relative to their littermate controls, which could be recapitulated in cultured brain ECs from these mice. Nuclear levels of TDP-43 were reduced in vitro and in vivo in ECs from TARDBP G348C mice. This coincided with a reduction in junctional proteins VE-cadherin, claudin-5 and ZO-1 in isolated ECs, supporting a cell autonomous effect on barrier function through a loss of nuclear TDP-43. We further examined two models of Tardbp deletion in ECs, and found that the loss of TDP-43 throughout the endothelium led to systemic endothelial activation and permeability. Deletion specifically within the brain endothelium acutely increased BBB permeability, and eventually led to hallmarks of FTD, including fibrin deposition, microglial and astrocyte activation, and behavioral defects. Together, these data show that TDP-43 dysfunction specifically within brain ECs would contribute to the BBB defects observed early in the progression of ALS/FTLD.
Figures



References
-
- Tziortzouda P, Van Den Bosch L, Hirth F. Triad of TDP43 control in neurodegeneration: autoregulation, localization and aggregation. Nat Rev Neurosci. 2021. Apr;22(4):197–208. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
