This is a preprint.
Genome-wide CRISPRi Screen in Human iNeurons to Identify Novel Focal Cortical Dysplasia Genes
- PMID: 38168415
- PMCID: PMC10760100
- DOI: 10.1101/2023.12.13.571474
Genome-wide CRISPRi Screen in Human iNeurons to Identify Novel Focal Cortical Dysplasia Genes
Abstract
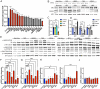
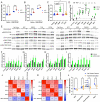
Focal cortical dysplasia (FCD) is a common cause of focal epilepsy that typically results from brain mosaic mutations in the mTOR cell signaling pathway. To identify new FCD genes, we developed an in vitro CRISPRi screen in human neurons and used FACS enrichment based on the FCD biomarker, phosphorylated S6 ribosomal protein (pS6). Using whole-genome (110,000 gRNAs) and candidate (129 gRNAs) libraries, we discovered 12 new genes that significantly increase pS6 levels. Interestingly, positive hits were enriched for brain-specific genes, highlighting the effectiveness of using human iPSC-derived induced neurons (iNeurons) in our screen. We investigated the signaling pathways of six candidate genes: LRRC4, EIF3A, TSN, HIP1, PIK3R3, and URI1. All six genes increased phosphorylation of S6. However, only two genes, PIK3R3 and HIP1, caused hyperphosphorylation more proximally in the AKT/mTOR/S6 signaling pathway. Importantly, these two genes have recently been found independently to be mutated in resected brain tissue from FCD patients, supporting the predictive validity of our screen. Knocking down each of the other four genes (LRRC4, EIF3A, TSN, and URI1) in iNeurons caused them to become resistant to the loss of growth factor signaling; without growth factor stimulation, pS6 levels were comparable to growth factor stimulated controls. Our data markedly expand the set of genes that are likely to regulate mTOR pathway signaling in neurons and provide additional targets for identifying somatic gene variants that cause FCD.
Keywords: Biological sciences; CRISPRi screen; Neuroscience/Genetics; epilepsy; focal cortical dysplasia; iNeuron; mTOR.
Conflict of interest statement
Competing Interest Statement: The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


References
-
- Engel J., Pedley T. A., Aicardi J., Epilepsy: a comprehensive textbook (Lippincott Williams & Wilkins, 2008), vol. 3.
-
- Bast T., Ramantani G., Seitz A., Rating D., Focal cortical dysplasia: prevalence, clinical presentation and epilepsy in children and adults. Acta Neurologica Scandinavica 113, 72–81 (2006). - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
