This is a preprint.
A molecular toolkit for heterologous protein secretion across Bacteroides species
- PMID: 38168418
- PMCID: PMC10760143
- DOI: 10.1101/2023.12.14.571725
A molecular toolkit for heterologous protein secretion across Bacteroides species
Update in
-
A molecular toolkit for heterologous protein secretion across Bacteroides species.Nat Commun. 2024 Nov 11;15(1):9741. doi: 10.1038/s41467-024-53845-7. Nat Commun. 2024. PMID: 39528443 Free PMC article.
Abstract
Bacteroides species are abundant and prevalent stably colonizing members of the human gut microbiota, making them a promising chassis for developing long-term interventions for chronic diseases. Engineering these bacteria as on-site production and delivery vehicles for biologic drugs or diagnostics, however, requires efficient heterologous protein secretion tools, which are currently lacking. To address this limitation, we systematically investigated methods to enable heterologous protein secretion in Bacteroides using both endogenous and exogenous secretion systems. Here, we report a collection of secretion carriers that can export functional proteins across multiple Bacteroides species at high titers. To understand the mechanistic drivers of Bacteroides secretion, we characterized signal peptide sequence features as well as post-secretion extracellular fate and cargo size limit of protein cargo. To increase titers and enable flexible control of protein secretion, we developed a strong, self-contained, inducible expression circuit. Finally, we validated the functionality of our secretion carriers in vivo in a mouse model. This toolkit should enable expanded development of long-term living therapeutic interventions for chronic gastrointestinal disease.
Keywords: Bacteroides; antibody fragments; bacterial therapeutics; commensal bacteria; drug delivery; live biotherapeutic products; outer membrane vesicles; protein secretion; synthetic biology; therapeutic proteins.
Conflict of interest statement
Declaration of interests Y.H.Y. and S.J.S. have filed a patent application on this work (PCT/US2023/083131). The authors declare no other competing interests.
Figures


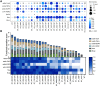
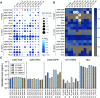



Similar articles
-
A molecular toolkit for heterologous protein secretion across Bacteroides species.Nat Commun. 2024 Nov 11;15(1):9741. doi: 10.1038/s41467-024-53845-7. Nat Commun. 2024. PMID: 39528443 Free PMC article.
-
Streamlined Genetic Manipulation of Diverse Bacteroides and Parabacteroides Isolates from the Human Gut Microbiota.mBio. 2019 Aug 13;10(4):e01762-19. doi: 10.1128/mBio.01762-19. mBio. 2019. PMID: 31409684 Free PMC article.
-
Engineering the human gut commensal Bacteroides thetaiotaomicron with synthetic biology.Curr Opin Chem Biol. 2022 Oct;70:102178. doi: 10.1016/j.cbpa.2022.102178. Epub 2022 Jun 24. Curr Opin Chem Biol. 2022. PMID: 35759819 Review.
-
Advances in synthetic biology toolboxes paving the way for mechanistic understanding and strain engineering of gut commensal Bacteroides spp. and Clostridium spp.Biotechnol Adv. 2023 Dec;69:108272. doi: 10.1016/j.biotechadv.2023.108272. Epub 2023 Oct 14. Biotechnol Adv. 2023. PMID: 37844770 Review.
-
Microbiota and Pathogen Proteases Modulate Type III Secretion Activity in Enterohemorrhagic Escherichia coli.mBio. 2018 Dec 4;9(6):e02204-18. doi: 10.1128/mBio.02204-18. mBio. 2018. PMID: 30514785 Free PMC article.
References
-
- Isabella V.M., Ha B.N., Castillo M.J., Lubkowicz D.J., Rowe S.E., Millet Y.A., Anderson C.L., Li N., Fisher A.B., West K.A., et al. (2018). Development of a synthetic live bacterial therapeutic for the human metabolic disease phenylketonuria. Nat Biotechnol 36, 857–864. 10.1038/nbt.4222. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
