This is a preprint.
Rescue of Impaired Blood-Brain Barrier in Tuberous Sclerosis Complex Patient Derived Neurovascular Unit
- PMID: 38168450
- PMCID: PMC10760190
- DOI: 10.1101/2023.12.15.571738
Rescue of Impaired Blood-Brain Barrier in Tuberous Sclerosis Complex Patient Derived Neurovascular Unit
Update in
-
Rescue of impaired blood-brain barrier in tuberous sclerosis complex patient derived neurovascular unit.J Neurodev Disord. 2024 May 23;16(1):27. doi: 10.1186/s11689-024-09543-y. J Neurodev Disord. 2024. PMID: 38783199 Free PMC article.
Abstract
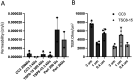
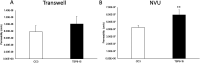
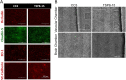
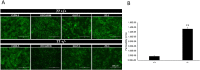
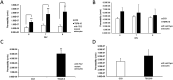
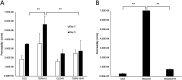
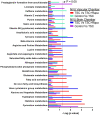
Tuberous sclerosis complex (TSC) is a multi-system genetic disease that causes benign tumors in the brain and other vital organs. The most debilitating symptoms result from involvement of the central nervous system and lead to a multitude of severe symptoms including seizures, intellectual disability, autism, and behavioral problems. TSC is caused by heterozygous mutations of either the TSC1 or TSC2 gene. Dysregulation of mTOR kinase with its multifaceted downstream signaling alterations is central to disease pathogenesis. Although the neurological sequelae of the disease are well established, little is known about how these mutations might affect cellular components and the function of the blood-brain barrier (BBB). We generated disease-specific cell models of the BBB by leveraging human induced pluripotent stem cell and microfluidic cell culture technologies. Using these microphysiological systems, we demonstrate that the BBB generated from TSC2 heterozygous mutant cells shows increased permeability which can be rescued by wild type astrocytes and with treatment with rapamycin, an mTOR kinase inhibitor. Our results further demonstrate the utility of microphysiological systems to study human neurological disorders and advance our knowledge of the cell lineages contributing to TSC pathogenesis.
Keywords: BBB; astrocytes; human stem cells; mTOR; microfluidics; rapamycin; tissue chips.
Figures


Similar articles
-
Rescue of impaired blood-brain barrier in tuberous sclerosis complex patient derived neurovascular unit.J Neurodev Disord. 2024 May 23;16(1):27. doi: 10.1186/s11689-024-09543-y. J Neurodev Disord. 2024. PMID: 38783199 Free PMC article.
-
A circuitry and biochemical basis for tuberous sclerosis symptoms: from epilepsy to neurocognitive deficits.Int J Dev Neurosci. 2013 Nov;31(7):667-78. doi: 10.1016/j.ijdevneu.2013.02.008. Epub 2013 Feb 26. Int J Dev Neurosci. 2013. PMID: 23485365 Free PMC article. Review.
-
Tuberous Sclerosis Complex (TSC) Inactivation Increases Neuronal Network Activity by Enhancing Ca2+ Influx via L-Type Ca2+ Channels.J Neurosci. 2021 Sep 29;41(39):8134-8149. doi: 10.1523/JNEUROSCI.1930-20.2021. Epub 2021 Aug 20. J Neurosci. 2021. PMID: 34417327 Free PMC article.
-
Biallelic Mutations in TSC2 Lead to Abnormalities Associated with Cortical Tubers in Human iPSC-Derived Neurons.J Neurosci. 2019 Nov 20;39(47):9294-9305. doi: 10.1523/JNEUROSCI.0642-19.2019. Epub 2019 Oct 7. J Neurosci. 2019. PMID: 31591157 Free PMC article.
-
Brain Symptoms of Tuberous Sclerosis Complex: Pathogenesis and Treatment.Int J Mol Sci. 2021 Jun 22;22(13):6677. doi: 10.3390/ijms22136677. Int J Mol Sci. 2021. PMID: 34206526 Free PMC article. Review.
References
-
- Au K. S., Williams A. T., Roach E. S., Batchelor L., Sparagana S. P., Delgado M. R., Wheless J. W., Baumgartner J. E., Roa B. B., Wilson C. M., Smith-Knuppel T. K., Cheung M. Y., Whittemore V. H., King T. M. and Northrup H. (2007). "Genotype/phenotype correlation in 325 individuals referred for a diagnosis of tuberous sclerosis complex in the United States." Genet Med 9(2): 88–100. - PubMed
-
- Baybis M., Yu J., Lee A., Golden J. A., Weiner H., McKhann G. 2nd, Aronica E. and Crino P. B. (2004). "mTOR cascade activation distinguishes tubers from focal cortical dysplasia." Ann Neurol 56(4): 478–487. - PubMed
-
- Bissler J. J., Kingswood J. C., Radzikowska E., Zonnenberg B. A., Frost M., Belousova E., Sauter M., Nonomura N., Brakemeier S., de Vries P. J., Whittemore V. H., Chen D., Sahmoud T., Shah G., Lincy J., Lebwohl D. and Budde K. (2013). "Everolimus for angiomyolipoma associated with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis (EXIST-2): a multicentre, randomised, double-blind, placebo-controlled trial." Lancet 381(9869): 817–824. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
