Use of smartphone-based remote assessments of multiple sclerosis in Floodlight Open, a global, prospective, open-access study
- PMID: 38168498
- PMCID: PMC10762023
- DOI: 10.1038/s41598-023-49299-4
Use of smartphone-based remote assessments of multiple sclerosis in Floodlight Open, a global, prospective, open-access study
Abstract
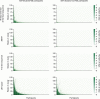
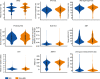
Floodlight Open was a global, open-access, digital-only study designed to understand the drivers and barriers in deployment and use of a smartphone app in a naturalistic setting and broad study population of people with and without multiple sclerosis (MS). The study utilised the Floodlight Open app: a 'bring-your-own-device' solution that remotely measures a user's mood, cognition, hand motor function, and gait and postural stability via smartphone sensor-based tests requiring active user input ('active tests'). Levels of mobility of study participants ('life-space measurement') were passively measured. Study data from these tests were made available via an open-access platform. Data from 1350 participants with self-declared MS and 1133 participants with self-declared non-MS from 17 countries across four continents were included in this report. Overall, MS participants provided active test data for a mean duration of 5.6 weeks or a mean duration of 19 non-consecutive days. This duration increased among MS participants who persisted beyond the first week to a mean of 10.3 weeks or 36.5 non-consecutive days. Passively collected life-space measurement data were generated by MS participants for a mean duration of 9.8 weeks or 50.6 non-consecutive days. This duration increased to 16.3 weeks/85.1 non-consecutive days among MS participants who persisted beyond the first week. Older age, self-declared MS disease status, and clinical supervision as part of concomitant clinical research were all significantly associated with higher persistence of the use of the Floodlight Open app. MS participants performed significantly worse than non-MS participants on four out of seven active tests. The findings from this multinational study inform future research to improve the dynamics of persistence of use of digital monitoring tools and further highlight challenges and opportunities in applying them to support MS clinical care.
© 2023. The Author(s).
Conflict of interest statement
The authors declare the following competing financial interests and no competing non-financial interests: J Oh has received personal compensation for consulting or speaking from EMD Serono, Sanofi-Genzyme, Biogen Idec., F. Hoffmann-La Roche Ltd., Celgene, and Novartis; and has received research funding from the MS Society of Canada, National MS Society, Brain Canada, Biogen Idec., and Sanofi-Genzyme. L Capezzuto is an employee of F. Hoffmann-La Roche Ltd. L Kriara is an employee of F. Hoffmann-La Roche Ltd. J Schjodt-Eriksen is an employee of and shareholder in F. Hoffmann-La Roche Ltd. J van Beek is an employee of Biogen Inc. C Bernasconi received consulting fees as a contractor for F. Hoffmann-La Roche Ltd. during the execution of this work. X Montalban has received speaking honoraria and travel expenses for participation in scientific meetings, has been a steering committee member of clinical trials, or participated in advisory boards of clinical trials in the past years with Actelion, Alexion, Bayer, Biogen, Celgene, EMD Serono, Genzyme, Immunic, MedDay, Merck, Mylan, Nervgen, Novartis, Roche, Sanofi-Genzyme, Teva Pharmaceuticals, TG Therapeutics, Excemed, MSIF, and NMSSH. H Butzkueven’s institution (Monash University) has received funding from Biogen, F. Hoffmann-La Roche Ltd., Merck, and Novartis; has carried out contracted research for Novartis, Merck, F. Hoffmann-La Roche Ltd., and Biogen; has taken part in speaker’s bureaus for Biogen, Genzyme, F. Hoffmann-La Roche Ltd., and Merck; and has received personal grants from Oxford Pharmagenesis and Biogen. L Kappos’ institutions (University Hospital Basel/Stiftung Neuroimmunology and Neuroscience Basel) have received and used exclusively for research support payments for steering committee and advisory board participation, consultancy services, and participation in educational activities from: Actelion, Aurigia Vision AG, Bayer, Bristol Myers Squibb, df-mp Molnia & Pohlmann, Celgene, Eli Lilly, EMD Serono, Genentech, GlaxoSmithKline, Janssen, Japan Tobacco, Merck, MH Consulting, Minoryx, Novartis, Österreichische Gesellschaft für Neurologie, F. Hoffmann-La Roche Ltd., Senda Biosciences Inc., Sanofi, Santhera, Shionogi BV, TG Therapeutics, and Wellmera; and license fees for Neurostatus-UHB products; grants from Novartis, Innosuisse, and F. Hoffmann-La Roche Ltd. G Giovannoni received personal compensation in the past for serving as a consultant for AbbVie, Actelion, Atara Bio, Biogen, Celgene, Sanofi-Genzyme, Genentech, GlaxoSmithKline, Merck-Serono, Novartis, Roche, and Teva Pharmaceuticals; has received personal compensation from Elsevier for serving as an editor on
Figures



Similar articles
-
Investigating Measurement Equivalence of Smartphone Sensor-Based Assessments: Remote, Digital, Bring-Your-Own-Device Study.J Med Internet Res. 2025 Apr 3;27:e63090. doi: 10.2196/63090. J Med Internet Res. 2025. PMID: 40179369 Free PMC article.
-
User Experience of a Large-Scale Smartphone-Based Observational Study in Multiple Sclerosis: Global, Open-Access, Digital-Only Study.JMIR Hum Factors. 2024 Sep 11;11:e57033. doi: 10.2196/57033. JMIR Hum Factors. 2024. PMID: 39259964 Free PMC article.
-
Evaluating the Utility of Smartphone-Based Sensor Assessments in Persons With Multiple Sclerosis in the Real-World Using an App (elevateMS): Observational, Prospective Pilot Digital Health Study.JMIR Mhealth Uhealth. 2020 Oct 27;8(10):e22108. doi: 10.2196/22108. JMIR Mhealth Uhealth. 2020. PMID: 33107827 Free PMC article.
-
Adherence and Satisfaction of Smartphone- and Smartwatch-Based Remote Active Testing and Passive Monitoring in People With Multiple Sclerosis: Nonrandomized Interventional Feasibility Study.J Med Internet Res. 2019 Aug 30;21(8):e14863. doi: 10.2196/14863. J Med Internet Res. 2019. PMID: 31471961 Free PMC article.
-
Smartphones for musculoskeletal research - hype or hope? Lessons from a decennium of mHealth studies.BMC Musculoskelet Disord. 2022 May 23;23(1):487. doi: 10.1186/s12891-022-05420-8. BMC Musculoskelet Disord. 2022. PMID: 35606783 Free PMC article. Review.
Cited by
-
Longitudinal Digital Phenotyping of Multiple Sclerosis Severity Using Passively Sensed Behaviors and Ecological Momentary Assessments.medRxiv [Preprint]. 2024 Dec 8:2024.11.02.24316647. doi: 10.1101/2024.11.02.24316647. medRxiv. 2024. Update in: J Med Internet Res. 2025 Jun 3;27:e70871. doi: 10.2196/70871. PMID: 39677484 Free PMC article. Updated. Preprint.
-
Smartphone postural sway and pronator drift tests as measures of neurological disability.BMC Neurol. 2025 Feb 5;25(1):50. doi: 10.1186/s12883-025-04038-2. BMC Neurol. 2025. PMID: 39910508 Free PMC article.
-
The Canadian Prospective Cohort Study to understand progression in multiple sclerosis: baseline characteristics.Ther Adv Neurol Disord. 2024 Sep 12;17:17562864241273045. doi: 10.1177/17562864241273045. eCollection 2024. Ther Adv Neurol Disord. 2024. PMID: 39282637 Free PMC article.
-
Digital outcome measures are associated with brain atrophy in patients with multiple sclerosis.J Neurol. 2024 Sep;271(9):5958-5968. doi: 10.1007/s00415-024-12516-9. Epub 2024 Jul 15. J Neurol. 2024. PMID: 39008036 Free PMC article.
-
[Digital health apps : How to achieve the successful integration into healthcare].Inn Med (Heidelb). 2024 Dec;65(12):1261-1265. doi: 10.1007/s00108-024-01774-4. Epub 2024 Oct 10. Inn Med (Heidelb). 2024. PMID: 39387860 Free PMC article. Review. German.
References
-
- Kappos L, et al. Contribution of relapse-independent progression vs relapse-associated worsening to overall confirmed disability accumulation in typical relapsing multiple sclerosis in a pooled analysis of 2 randomized clinical trials. JAMA Neurol. 2020;77:1132–1140. doi: 10.1001/jamaneurol.2020.1568. - DOI - PMC - PubMed

