Effects of time-restricted exercise on activity rhythms and exercise-induced adaptations in the heart
- PMID: 38168503
- PMCID: PMC10761674
- DOI: 10.1038/s41598-023-50113-4
Effects of time-restricted exercise on activity rhythms and exercise-induced adaptations in the heart
Abstract
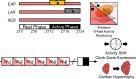
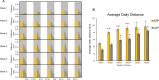
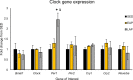
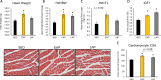
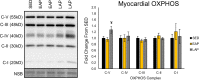
Circadian rhythms play a crucial role in the regulation of various physiological processes, including cardiovascular function and metabolism. Exercise provokes numerous beneficial adaptations in heart, including physiological hypertrophy, and serves to shift circadian rhythms. This study investigated the impact of time-restricted exercise training on exercise-induced adaptations in the heart and locomotor activity rhythms. Male mice (n = 45) were allocated to perform voluntary, time-restricted exercise in the early active phase (EAP), late active phase (LAP), or remain sedentary (SED) for 6 weeks. Subsequently, mice were allowed 24-h ad libitum access to the running wheel to assess diurnal rhythms in locomotor activity. Heart weight and cross-sectional area were measured at sacrifice, and cardiac protein and gene expression levels were assessed for markers of mitochondrial abundance and circadian clock gene expression. Mice rapidly adapted to wheel running, with EAP mice exhibiting a significantly greater running distance compared to LAP mice. Time-restricted exercise induced a shift in voluntary wheel activity during the 24-h free access period, with the acrophase in activity being significantly earlier in EAP mice compared to LAP mice. Gene expression analysis revealed a higher expression of Per1 in LAP mice. EAP exercise elicited greater cardiac hypertrophy compared to LAP exercise. These findings suggest that the timing of exercise affects myocardial adaptations, with exercise in the early active phase inducing hypertrophy in the heart. Understanding the time-of-day dependent response to exercise in the heart may have implications for optimizing exercise interventions for cardiovascular health.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Social jet lag impairs exercise volume and attenuates physiological and metabolic adaptations to voluntary exercise training.J Appl Physiol (1985). 2024 Apr 1;136(4):996-1006. doi: 10.1152/japplphysiol.00632.2023. Epub 2024 Mar 7. J Appl Physiol (1985). 2024. PMID: 38450426 Free PMC article.
-
Combining Time-Restricted Wheel Running and Feeding During the Light Phase Increases Running Intensity Under High-Fat Diet Conditions Without Altering the Total Amount of Daily Running.Int J Mol Sci. 2025 Aug 7;26(15):7658. doi: 10.3390/ijms26157658. Int J Mol Sci. 2025. PMID: 40806786 Free PMC article.
-
Mood Status Response to Physical Activity and Its Influence on Performance: Are Chronotype and Exercise Timing Affect?Int J Environ Res Public Health. 2023 Feb 5;20(4):2822. doi: 10.3390/ijerph20042822. Int J Environ Res Public Health. 2023. PMID: 36833520 Free PMC article.
-
Social jetlag alters markers of exercise-induced mitochondrial adaptations in the heart.NPJ Biol Timing Sleep. 2025;2(1):4. doi: 10.1038/s44323-024-00019-9. Epub 2025 Jan 25. NPJ Biol Timing Sleep. 2025. PMID: 40838132 Free PMC article.
-
Effects of Chrono-Exercise and Chrono-Nutrition on Muscle Health: Understanding the Molecular Mechanisms Activated by Timed Exercise and Consumption of Proteins and Carbohydrates.Nutr Rev. 2025 Aug 1;83(8):1571-1593. doi: 10.1093/nutrit/nuaf007. Nutr Rev. 2025. PMID: 40048668 Free PMC article. Review.
Cited by
-
Circadian clock and its effect on aging and lifespan.Biogerontology. 2025 Jun 27;26(4):132. doi: 10.1007/s10522-025-10281-4. Biogerontology. 2025. PMID: 40576851 Review.
-
Chronic circadian disruption in adolescent mice impairs hippocampal memory disrupting gene expression oscillations.Sci Rep. 2025 Jul 19;15(1):26291. doi: 10.1038/s41598-025-12237-7. Sci Rep. 2025. PMID: 40684045 Free PMC article.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

