Reparation of nano-FeS by ultrasonic precipitation for treatment of acidic chromium-containing wastewater
- PMID: 38168529
- PMCID: PMC10761992
- DOI: 10.1038/s41598-023-50070-y
Reparation of nano-FeS by ultrasonic precipitation for treatment of acidic chromium-containing wastewater
Abstract
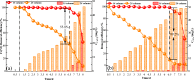
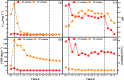
Nano-FeS is prone to agglomeration in the treatment of chromium-containing wastewater, and ultrasonic precipitation was used to synthesize nano-FeS to increase its dispersion. The optimization of the preparation method was carried out by single factor method (reaction temperature, Fe/S molar ratio and FeSO4 dropping flow rate) and response surface methodology. Dynamic experiments were constructed to investigate the long-term remediation effect and water column changes of nano-FeS and its solid particles. The changes of the remediation materials before and after the reaction were observed by SEM, and the mechanism of the remediation of chromium-containing wastewater by nano-FeS prepared by ultrasonication was revealed by XRD. The results showed that the reaction temperature of 12 °C, Fe/S molar ratio of 3.5 and FeSO4 dropping flow rate of 0.5 mL/s were the best parameters for the preparation of nano-FeS. The nano-FeS has efficient dispersion and well-defined mesoporous structure in the form of needles and whiskers of 40-80 nm. The dynamic experiments showed that the average removal of Cr(VI) and total chromium by nano-FeS and its immobilized particles were 94.97% and 63.51%, 94.93% and 45.76%, respectively. Fe2+ and S2- ionized by the FeS nanoparticles rapidly reduced Cr(VI) to Cr(III). Part of S2- may reduce Fe3+ to Fe2+, forming a small iron cycle that gradually decreases with the ion concentration. Cr(III) and Fe2+ form Cr(OH)3 and FeOOH, respectively, with the change of aqueous environment. Another part of S2- reacts with Cr(III) to form Cr2S3 precipitate or is oxidized to singlet sulfur. The FeS nanoparticles change from short rod-shaped to spherical shape. Compared with the conventional chemical precipitation method, the method used in this study is simple, low cost, small particle size and high removal rate per unit.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Li, T. D. Preparation of Nano FeS and Its Treatment of Acidic Chromium Containing Wastewater. Liaoning Technical University. 10.27210/d.cnki.glnju.2019.000498 (2019)
-
- Shi Z, et al. Cof tzda/ag/agbr z-scheme heterojunction photocatalyst for efficient visible light driven elimination of antibiotics tetracycline and heavy metal ion Cr(VI) Separat. Purif. Technol. 2022;288:120717. doi: 10.1016/j.seppur.2022.120717. - DOI
-
- Li LX, et al. Synthesis of carnation flower-like Bi2O2CO3 photocatalyst and its promising application for photoreduction of Cr(VI) Adv. Powder Technol. 2022;33:103481. doi: 10.1016/j.apt.2022.103481. - DOI
-
- Zhao Q, et al. Photocatalytic Cr(VI) reduction over mil-101(Fe)-NH2 immobilized on alumina substrate from batch test to continuous operation. Chem. Eng. J. 2022;3:429. doi: 10.1016/j.cej.2021.132497. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

