Prospective study of an adalimumab combined with partial enteral nutrition in the induction period of Crohn's disease
- PMID: 38168701
- PMCID: PMC10824800
- DOI: 10.1007/s00011-023-01828-7
Prospective study of an adalimumab combined with partial enteral nutrition in the induction period of Crohn's disease
Abstract
Background: Adalimumab monotherapy can suppress gut inflammation and induce remission in active Crohn's disease but has some limitations. Exclusive enteral nutrition (EEN) is recommended for patients with mild to moderate Crohn's disease (CD), but implementation is challenging.
Aim: To evaluate the effectiveness of adalimumab combined with partial enteral nutrition (PEN) in the induction therapy for Crohn's disease.
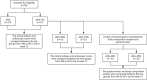
Methods: A prospective cohort study was designed and a total of 56 patients with active CD who met the criteria for enteral nutrition (EN) treatment in our hospital were selected. The baseline data of all patients were collected including age, sex and other general information. The changes in fecal calprotectin, C-reactive protein (CRP), albumin(Alb), hemoglobin (Hb), platelets (Plt), erythrocyte sedimentation rate (ESR), Crohn's disease activity index score (CDAI), simple endoscopic score (SES-CD) and body mass index (BMI) were compared between the adalimumab combined with enteral nutrition (ADA+EN) group (N = 37) the adalimumab group (ADA) (N = 19) at week 0 (W0) and treatment outcomes at week 12(W12). Additionally, the differences between the two groups before and after treatment were evaluated. Then the ADA+EN group was divided into an adalimumab combined with exclusive enteral nutrition subgroup (ADA+EEN) and an adalimumab combined with partial nutrition subgroup (ADA+PEN) according to enteral nutrition intake. The changes in fecal calprotectin, CRP, Alb, Hb, Plt, ESR and CDAI, SES-CD and BMI were compared between the ADA+EEN group and the ADA+PEN group at week 0 (W0) and treatment outcomes at week 12(W12). The differences between the two groups before and after treatment were evaluated. To evaluate the effectiveness of the two treatments on patients' quality of life, nutritional recovery and body composition, patients in the ADA+EN group were needed to complete the Inflammatory Bowel Disease Questionnaire (IBDQ), EQ-5D-5L, the EuroQol visual analogue scale (EQ-VAS) and body composition analysis.A total of 28 patients completed all questionnaires and body composition analyses at week 0 and week 12, including 10 patients in the ADA+EEN group and 18 patients in the ADA+PEN group, respectively. The differences of in IBDQ, EQ-5D-5L and body composition analysis were compared between the two groups at week 0 (W0) and treatment outcomes at week 12(W12). Additionally, the differences between the two groups before and after treatment were evaluated.
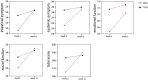
Results: These investigated indexes such as calprotectin, Hb, Plt, ESR, Alb, BMI, CRP, CDAI and SES-CD scores were significantly different before and after treatment in the ADA+EN group (p < 0.01). However, fecal calprotectin, Hb, SES-CD scores and Alb in the ADA group were not statistically significantly different from W0 to W12 (p > 0.05). The fecal calprotectin and CDAI scores in the ADA+EN group were significantly lower than those in the ADA group after treatment. The differences in all factors before and after treatment between the ADA+PEN group and the ADA+EEN group were statistically significant (p < 0.05). However, there was no significant difference between the two groups at week 12 (p > 0.05).
Conclusion: Adalimumab combined with EN are more effective than ADA monotherapy in terms of endoscopy and clinical remission. By comparing the investigated indicators such as calprotectin, Hb, Plt, ESR ,CRP and SES-CD scores, it was proven that adalimumab combined with partial enteral nutrition or exclusive enteral nutrition has the same remission effect in induced Crohn's disease. The combination of biological agents and partial nutrition can improve medical order compliance, psychological burden and quality of life. Therefore, adalimumab combined with partial nutrition can be used as the first-line treatment for CD induced remission.
Keywords: Adalimumab; Crohn’s disease; Exclusive enteral nutrition; Partial enteral nutrition.
© 2024. The Author(s).
Conflict of interest statement
The Author(s) declare(s) that there is no conflict of interest.
Figures
Similar articles
-
Treatment of Active Crohn's Disease in Children Using Partial Enteral Nutrition Combined with a Modified Crohn's Disease Exclusion Diet: A Pilot Prospective Cohort Trial on Clinical and Endoscopic Outcomes.Nutrients. 2023 Nov 4;15(21):4676. doi: 10.3390/nu15214676. Nutrients. 2023. PMID: 37960328 Free PMC article.
-
Partial enteral nutrition induces clinical and endoscopic remission in active pediatric Crohn's disease: results of a prospective cohort study.Eur J Pediatr. 2020 Mar;179(3):431-438. doi: 10.1007/s00431-019-03520-7. Epub 2019 Nov 28. Eur J Pediatr. 2020. PMID: 31781933
-
Crohn's Disease Exclusion Diet Plus Partial Enteral Nutrition Induces Sustained Remission in a Randomized Controlled Trial.Gastroenterology. 2019 Aug;157(2):440-450.e8. doi: 10.1053/j.gastro.2019.04.021. Epub 2019 Jun 4. Gastroenterology. 2019. PMID: 31170412 Clinical Trial.
-
Cannabis for the treatment of Crohn's disease.Cochrane Database Syst Rev. 2018 Nov 8;11(11):CD012853. doi: 10.1002/14651858.CD012853.pub2. Cochrane Database Syst Rev. 2018. PMID: 30407616 Free PMC article.
-
Review of exclusive enteral therapy in adult Crohn's disease.BMJ Open Gastroenterol. 2021 Sep;8(1):e000745. doi: 10.1136/bmjgast-2021-000745. BMJ Open Gastroenterol. 2021. PMID: 34580154 Free PMC article. Review.
Cited by
-
Patient experiences with and adherence to Crohn's disease exclusion diet in Dutch Crohn's disease patients: a cohort study.Therap Adv Gastroenterol. 2025 Mar 12;18:17562848251323553. doi: 10.1177/17562848251323553. eCollection 2025. Therap Adv Gastroenterol. 2025. PMID: 40078328 Free PMC article.
-
The efficacy of infliximab combined with partial enteral nutrition in the treatment of Crohn's disease: a cohort study.Front Nutr. 2025 Jun 18;12:1591954. doi: 10.3389/fnut.2025.1591954. eCollection 2025. Front Nutr. 2025. PMID: 40607024 Free PMC article.
-
Refractory Crohn's Disease: Perspectives, Unmet Needs and Innovations.Clin Exp Gastroenterol. 2024 Oct 10;17:261-315. doi: 10.2147/CEG.S434014. eCollection 2024. Clin Exp Gastroenterol. 2024. PMID: 39403342 Free PMC article. Review.
-
Exploring diet-microbiota interactions and therapeutic nutrition management in inflammatory bowel disease.Biophys Rep. 2025 Jun 30;11(3):180-197. doi: 10.52601/bpr.2024.240050. Biophys Rep. 2025. PMID: 40612235 Free PMC article.
-
The Application and Mechanism Analysis of Enteral Nutrition in Clinical Management of Chronic Diseases.Nutrients. 2025 Jan 26;17(3):450. doi: 10.3390/nu17030450. Nutrients. 2025. PMID: 39940308 Free PMC article.
References
-
- Rutgeerts P, Van Assche G, Sandborn WJ, et al. Adalimumab induces and maintains mucosal healing in patients with Crohn's disease: data from the EXTEND trial. Gastroenterology. 2012;142(5):1102–1111.e2. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

