A novel simple disposition index (SPINA-DI) from fasting insulin and glucose concentration as a robust measure of carbohydrate homeostasis
- PMID: 38169110
- PMCID: PMC11418405
- DOI: 10.1111/1753-0407.13525
A novel simple disposition index (SPINA-DI) from fasting insulin and glucose concentration as a robust measure of carbohydrate homeostasis
Abstract
Aims: The widely used dynamic disposition index, derived from oral glucose tolerance testing, is an integrative measure of the homeostatic performance of the insulin-glucose feedback control. Its collection is, however, time consuming and expensive. We, therefore, pursued the question if such a measure can be calculated at baseline/fasting conditions using plasma concentrations of insulin and glucose.
Methods: A new fasting-based disposition index (structure parameter inference approach-disposition index [SPINA-DI]) was calculated as the product of the reconstructed insulin receptor gain (SPINA-GR) times the secretory capacity of pancreatic beta cells (SPINA-GBeta). The novel index was evaluated in computer simulations and in three independent, multiethnic cohorts. The objectives were distribution in various populations, diagnostic performance, reliability and correlation to established physiological biomarkers of carbohydrate metabolism.
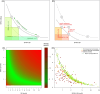
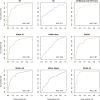
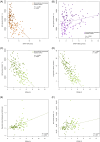
Results: Mathematical and in-silico analysis demonstrated SPINA-DI to mirror the hyperbolic relationship between insulin sensitivity and beta-cell function and to represent an optimum of the homeostatic control. It significantly correlates to the oral glucose tolerance test based disposition index and other important physiological parameters. Furthermore, it revealed higher discriminatory power for the diagnosis of (pre)diabetes and superior retest reliability than other static and dynamic function tests of glucose homeostasis.
Conclusions: SPINA-DI is a novel simple reliable and inexpensive marker of insulin-glucose homeostasis suitable for screening purposes and a wider clinical application.
Keywords: disposition index; dynamical compensation; insulin‐glucose homeostasis.
© 2024 The Author(s). Journal of Diabetes published by Ruijin Hospital, Shanghai Jiaotong University School of Medicine and John Wiley & Sons Australia, Ltd.
Figures
Similar articles
-
Oral disposition index predicts the development of future diabetes above and beyond fasting and 2-h glucose levels.Diabetes Care. 2009 Feb;32(2):335-41. doi: 10.2337/dc08-1478. Epub 2008 Oct 28. Diabetes Care. 2009. PMID: 18957530 Free PMC article.
-
SPINA Carb: a simple mathematical model supporting fast in-vivo estimation of insulin sensitivity and beta cell function.Sci Rep. 2022 Oct 21;12(1):17659. doi: 10.1038/s41598-022-22531-3. Sci Rep. 2022. PMID: 36271244 Free PMC article.
-
Impact of traits of metabolic syndrome on β-cell function and insulin resistance in normal fasting, normal glucose tolerant subjects.Metab Syndr Relat Disord. 2012 Oct;10(5):344-50. doi: 10.1089/met.2012.0040. Epub 2012 Jul 17. Metab Syndr Relat Disord. 2012. PMID: 22803772
-
Association of β-cell function and insulin sensitivity with fasting and 2-h plasma glucose in a large Chinese population.Diabetes Obes Metab. 2012 Feb;14(2):174-80. doi: 10.1111/j.1463-1326.2011.01504.x. Epub 2011 Nov 13. Diabetes Obes Metab. 2012. PMID: 21951345
-
Determining pancreatic β-cell compensation for changing insulin sensitivity using an oral glucose tolerance test.Am J Physiol Endocrinol Metab. 2014 Nov 1;307(9):E822-9. doi: 10.1152/ajpendo.00269.2014. Epub 2014 Sep 2. Am J Physiol Endocrinol Metab. 2014. PMID: 25184989 Free PMC article.
Cited by
-
Endotyping Insulin-Glucose Homeostasis in Hidradenitis Suppurativa: The Impact of Diabetes Mellitus and Inflammation.J Clin Med. 2025 Mar 21;14(7):2145. doi: 10.3390/jcm14072145. J Clin Med. 2025. PMID: 40217596 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

