Tafenoquine-Atovaquone Combination Achieves Radical Cure and Confers Sterile Immunity in Experimental Models of Human Babesiosis
- PMID: 38169301
- PMCID: PMC10786256
- DOI: 10.1093/infdis/jiad315
Tafenoquine-Atovaquone Combination Achieves Radical Cure and Confers Sterile Immunity in Experimental Models of Human Babesiosis
Abstract
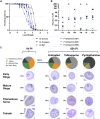
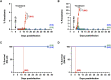
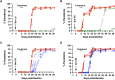
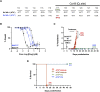
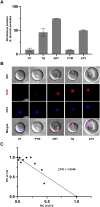
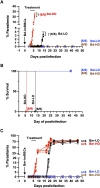
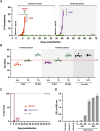
Human babesiosis is a potentially fatal tick-borne disease caused by intraerythrocytic Babesia parasites. The emergence of resistance to recommended therapies highlights the need for new and more effective treatments. Here we demonstrate that the 8-aminoquinoline antimalarial drug tafenoquine inhibits the growth of different Babesia species in vitro, is highly effective against Babesia microti and Babesia duncani in mice and protects animals from lethal infection caused by atovaquone-sensitive and -resistant B. duncani strains. We further show that a combination of tafenoquine and atovaquone achieves cure with no recrudescence in both models of human babesiosis. Interestingly, elimination of B. duncani infection in animals following drug treatment also confers immunity to subsequent challenge. Altogether, the data demonstrate superior efficacy of tafenoquine plus atovaquone combination over current therapies for the treatment of human babesiosis and highlight its potential in providing protective immunity against Babesia following parasite clearance.
Keywords: Babesia MO1; Babesia divergens; Babesia duncani; Babesia microti; Plasmodium falciparum; erythrocyte; human babesiosis; parasite; sterile immunity; tafenoquine.
© The Author(s) 2024. Published by Oxford University Press on behalf of Infectious Diseases Society of America. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures
Similar articles
-
Effectiveness of Two New Endochin-like Quinolones, ELQ-596 and ELQ-650, in Experimental Mouse Models of Human Babesiosis.ACS Infect Dis. 2024 Apr 12;10(4):1405-1413. doi: 10.1021/acsinfecdis.4c00143. Epub 2024 Apr 2. ACS Infect Dis. 2024. PMID: 38563132 Free PMC article.
-
Babesia duncani as a Model Organism to Study the Development, Virulence, and Drug Susceptibility of Intraerythrocytic Parasites In Vitro and In Vivo.J Infect Dis. 2022 Sep 28;226(7):1267-1275. doi: 10.1093/infdis/jiac181. J Infect Dis. 2022. PMID: 35512141 Free PMC article.
-
Effective Therapy Targeting Cytochrome bc1 Prevents Babesia Erythrocytic Development and Protects from Lethal Infection.Antimicrob Agents Chemother. 2021 Aug 17;65(9):e0066221. doi: 10.1128/AAC.00662-21. Epub 2021 Aug 17. Antimicrob Agents Chemother. 2021. PMID: 34152821 Free PMC article.
-
Treatment of Human Babesiosis: Then and Now.Pathogens. 2021 Sep 1;10(9):1120. doi: 10.3390/pathogens10091120. Pathogens. 2021. PMID: 34578153 Free PMC article. Review.
-
Human Babesiosis.Infect Dis Clin North Am. 2022 Sep;36(3):655-670. doi: 10.1016/j.idc.2022.02.009. Infect Dis Clin North Am. 2022. PMID: 36116841 Review.
Cited by
-
Persistent human babesiosis with low-grade parasitemia, challenges for clinical diagnosis and management.Heliyon. 2024 Nov 3;10(22):e39960. doi: 10.1016/j.heliyon.2024.e39960. eCollection 2024 Nov 30. Heliyon. 2024. PMID: 39634383 Free PMC article. Review.
-
Effectiveness of Two New Endochin-like Quinolones, ELQ-596 and ELQ-650, in Experimental Mouse Models of Human Babesiosis.ACS Infect Dis. 2024 Apr 12;10(4):1405-1413. doi: 10.1021/acsinfecdis.4c00143. Epub 2024 Apr 2. ACS Infect Dis. 2024. PMID: 38563132 Free PMC article.
-
Babesia duncani, a Model Organism for Investigating Intraerythrocytic Parasitism and Novel Antiparasitic Therapeutic Strategies.J Infect Dis. 2024 Jul 25;230(1):263-270. doi: 10.1093/infdis/jiae191. J Infect Dis. 2024. PMID: 39052743 Free PMC article. Review.
-
New insights in the diagnosis and treatment of equine piroplasmosis: pitfalls, idiosyncrasies, and myths.Front Vet Sci. 2024 Aug 14;11:1459989. doi: 10.3389/fvets.2024.1459989. eCollection 2024. Front Vet Sci. 2024. PMID: 39205808 Free PMC article. Review.
-
Babesia BdFE1 esterase is required for the anti-parasitic activity of the ACE inhibitor fosinopril.J Biol Chem. 2023 Nov;299(11):105313. doi: 10.1016/j.jbc.2023.105313. Epub 2023 Oct 4. J Biol Chem. 2023. PMID: 37797695 Free PMC article.
References
-
- Kjemtrup AM, Conrad PA. Human babesiosis: an emerging tick-borne disease. Int J Parasitol 2000; 30:1323–37. - PubMed
-
- Krause PJ. Human babesiosis. Int J Parasitol 2019; 49:165–74. - PubMed
-
- Krause PJ, Daily J, Telford SR, Vannier E, Lantos P, Spielman A. Shared features in the pathobiology of babesiosis and malaria. Trends Parasitol 2007; 23:605–10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

