Cellular zinc metabolism and zinc signaling: from biological functions to diseases and therapeutic targets
- PMID: 38169461
- PMCID: PMC10761908
- DOI: 10.1038/s41392-023-01679-y
Cellular zinc metabolism and zinc signaling: from biological functions to diseases and therapeutic targets
Abstract
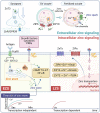
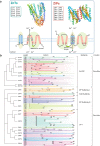
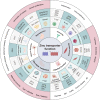
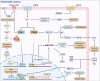
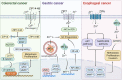
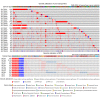
Zinc metabolism at the cellular level is critical for many biological processes in the body. A key observation is the disruption of cellular homeostasis, often coinciding with disease progression. As an essential factor in maintaining cellular equilibrium, cellular zinc has been increasingly spotlighted in the context of disease development. Extensive research suggests zinc's involvement in promoting malignancy and invasion in cancer cells, despite its low tissue concentration. This has led to a growing body of literature investigating zinc's cellular metabolism, particularly the functions of zinc transporters and storage mechanisms during cancer progression. Zinc transportation is under the control of two major transporter families: SLC30 (ZnT) for the excretion of zinc and SLC39 (ZIP) for the zinc intake. Additionally, the storage of this essential element is predominantly mediated by metallothioneins (MTs). This review consolidates knowledge on the critical functions of cellular zinc signaling and underscores potential molecular pathways linking zinc metabolism to disease progression, with a special focus on cancer. We also compile a summary of clinical trials involving zinc ions. Given the main localization of zinc transporters at the cell membrane, the potential for targeted therapies, including small molecules and monoclonal antibodies, offers promising avenues for future exploration.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

