Differential effect of cancer-associated fibroblast-derived extracellular vesicles on cisplatin resistance in oral squamous cell carcinoma via miR-876-3p
- PMID: 38169528
- PMCID: PMC10758057
- DOI: 10.7150/thno.87329
Differential effect of cancer-associated fibroblast-derived extracellular vesicles on cisplatin resistance in oral squamous cell carcinoma via miR-876-3p
Abstract
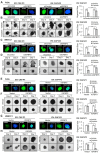
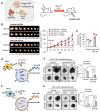
Rationale: Platinum-based chemotherapy is commonly used for treating solid tumors, but drug resistance often limits its effectiveness. Cancer-associated fibroblast (CAF)-derived extracellular vesicle (EV), which carry various miRNAs, have been implicated in chemotherapy resistance. However, the molecular mechanism through which CAFs modulate cisplatin resistance in oral squamous cell carcinoma (OSCC) is not well understood. We employed two distinct primary CAF types with differential impacts on cancer progression: CAF-P, representing a more aggressive cancer-promoting category, and CAF-D, characterized by properties that moderately delay cancer progression. Consequently, we sought to investigate whether the two CAF types differentially affect cisplatin sensitivity and the underlying molecular mechanism. Methods: The secretion profile was examined by utilizing an antibody microarray with conditioned medium obtained from the co-culture of OSCC cells and two types of primary CAFs. The effect of CAF-dependent factors on cisplatin resistance was investigated by utilizing conditioned media (CM) and extracellular vesicle (EVs) derived from CAFs. The impacts of candidate genes were confirmed using gain- and loss-of-function analyses in spheroids and organoids, and a mouse xenograft. Lastly, we compared the expression pattern of the candidate genes in tissues from OSCC patients exhibiting different responses to cisplatin. Results: When OSCC cells were cultured with conditioned media (CM) from the two different CAF groups, cisplatin resistance increased only under CAF-P CM. OSCC cells specifically expressed insulin-like growth factor binding protein 3 (IGFBP3) after co-culture with CAF-D. Meanwhile, IGFBP3-knockdown OSCC cells acquired cisplatin resistance in CAF-D CM. IGFBP3 expression was promoted by GATA-binding protein 1 (GATA1), a transcription factor targeted by miR-876-3p, which was enriched only in CAF-P-derived EV. Treatment with CAF-P EV carrying miR-876-3p antagomir decreased cisplatin resistance compared to control miRNA-carrying CAF-P EV. On comparing the staining intensity between cisplatin-sensitive and -insensitive tissues from OSCC patients, there was a positive correlation between IGFBP3 and GATA1 expression and cisplatin sensitivity in OSCC tissues from patients. Conclusion: These results provide insights for overcoming cisplatin resistance, especially concerning EVs within the tumor microenvironment. Furthermore, it is anticipated that the expression levels of GATA1 and miR-876-3p, along with IGFBP3, could aid in the prediction of cisplatin resistance.
Keywords: cancer-associated fibroblasts; cisplatin resistance; extracellular vesicles; hsa-miR-876-3p; insulin-like growth factor binding protein 3.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures







References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

