Quaternization drives spleen-to-lung tropism conversion for mRNA-loaded lipid-like nanoassemblies
- PMID: 38169552
- PMCID: PMC10758058
- DOI: 10.7150/thno.90071
Quaternization drives spleen-to-lung tropism conversion for mRNA-loaded lipid-like nanoassemblies
Abstract
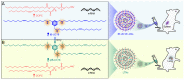
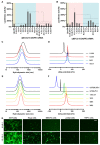
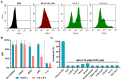
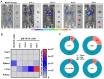
Background: As the overwhelming majority of advanced mRNA delivery systems are preferentially accumulated in the liver, there is an accelerating growth in the demand for the development of non-liver mRNA delivery platforms. Methods: In this study, we prepared cationic lipid-like nanoassemblies through a N-quaternizing strategy. Their physicochemical properties, in vitro mRNA delivery efficiency, and organ tropism in mice were investigated. Results: Introduction of quaternary ammonium groups onto lipid-like nanoassemblies not only enhances their mRNA delivery performance in vitro, but also completely alters their tropism from the spleen to the lung after intravenous administration in mice. Quaternized lipid-like nanoassemblies exhibit ultra-high specificity to the lung and are predominantly taken up by pulmonary immune cells, leading to over 95% of exogenous mRNA translation in the lungs. Such mRNA delivery carriers are stable even after more than one-year storage at ambient temperature. Conclusions: Quaternization provides an alternative method for design of new lung-targeted mRNA delivery systems without incorporation of targeting ligands, which should extend the therapeutic applicability of mRNA to lung diseases.
Keywords: lipid-like nanoassembly; lung targeting; quaternization; systemic mRNA delivery; ultra-high selectivity.
© The author(s).
Conflict of interest statement
Competing Interests: The authors declare the following financial interest which may be considered as potential competing interests: Y. Huang, J. Wu, S. Li, and B. Li are listed as inventors on an issued patent related to this work.
Figures
Similar articles
-
Cationic Lipid Pairs Enhance Liver-to-Lung Tropism of Lipid Nanoparticles for In Vivo mRNA Delivery.ACS Appl Mater Interfaces. 2024 May 22;16(20):25698-25709. doi: 10.1021/acsami.4c02415. Epub 2024 May 8. ACS Appl Mater Interfaces. 2024. PMID: 38717294
-
The replacement of helper lipids with charged alternatives in lipid nanoparticles facilitates targeted mRNA delivery to the spleen and lungs.J Control Release. 2022 May;345:819-831. doi: 10.1016/j.jconrel.2022.03.046. Epub 2022 Mar 26. J Control Release. 2022. PMID: 35346768 Free PMC article.
-
One-Component Cationic Lipids for Systemic mRNA Delivery to Splenic T Cells.Angew Chem Int Ed Engl. 2024 Jun 21;63(26):e202405444. doi: 10.1002/anie.202405444. Epub 2024 May 21. Angew Chem Int Ed Engl. 2024. PMID: 38637320
-
Preparation of selective organ-targeting (SORT) lipid nanoparticles (LNPs) using multiple technical methods for tissue-specific mRNA delivery.Nat Protoc. 2023 Jan;18(1):265-291. doi: 10.1038/s41596-022-00755-x. Epub 2022 Oct 31. Nat Protoc. 2023. PMID: 36316378 Free PMC article. Review.
-
Developing Biodegradable Lipid Nanoparticles for Intracellular mRNA Delivery and Genome Editing.Acc Chem Res. 2021 Nov 2;54(21):4001-4011. doi: 10.1021/acs.accounts.1c00500. Epub 2021 Oct 20. Acc Chem Res. 2021. PMID: 34668716 Review.
Cited by
-
Structure-guided design of endosomolytic chloroquine-like lipid nanoparticles for mRNA delivery and genome editing.Nat Commun. 2025 May 7;16(1):4241. doi: 10.1038/s41467-025-59501-y. Nat Commun. 2025. PMID: 40335474 Free PMC article.
-
Recent strategies for enhanced delivery of mRNA to the lungs.Nanomedicine (Lond). 2025 May;20(9):1043-1069. doi: 10.1080/17435889.2025.2485669. Epub 2025 Apr 7. Nanomedicine (Lond). 2025. PMID: 40190037 Review.
-
Lung-Specific mRNA Delivery Enabled by Sulfonium Lipid Nanoparticles.Nano Lett. 2024 Jul 3;24(26):8080-8088. doi: 10.1021/acs.nanolett.4c01854. Epub 2024 Jun 18. Nano Lett. 2024. PMID: 38888232 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

