Current evidence and therapeutic implication of PANoptosis in cancer
- PMID: 38169587
- PMCID: PMC10758053
- DOI: 10.7150/thno.91814
Current evidence and therapeutic implication of PANoptosis in cancer
Abstract
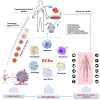
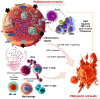
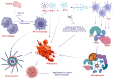
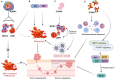
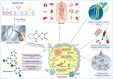
Regulated cell death (RCD) is considered a critical pathway in cancer therapy, contributing to eliminating cancer cells and influencing treatment outcomes. The application of RCD in cancer treatment is marked by its potential in targeted therapy and immunotherapy. As a type of RCD, PANoptosis has emerged as a unique form of programmed cell death (PCD) characterized by features of pyroptosis, apoptosis, and necroptosis but cannot be fully explained by any of these pathways alone. It is regulated by a multi-protein complex called the PANoptosome. As a relatively new concept first described in 2019, PANoptosis has been shown to play a role in many diseases, including cancer, infection, and inflammation. This study reviews the application of PCD in cancer, particularly the emergence and implication of PANoptosis in developing therapeutic strategies for cancer. Studies have shown that the characterization of PANoptosis patterns in cancer can predict survival and response to immunotherapy and chemotherapy, highlighting the potential for PANoptosis to be used as a therapeutic target in cancer treatment. It also plays a role in limiting the spread of cancer cells. PANoptosis allows for the elimination of cancer cells by multiple cell death pathways and has the potential to address various challenges in cancer treatment, including drug resistance and immune evasion. Moreover, active investigation of the mechanisms and potential therapeutic agents that can induce PANoptosis in cancer cells is likely to yield effective cancer treatments and improve patient outcomes. Research on PANoptosis is still ongoing, but it is a rapidly evolving field with the potential to lead to new treatments for various diseases, including cancer.
Keywords: PANoptosis; cancer; immunity; regulated cell death; therapy.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures


Similar articles
-
Implications of inflammatory cell death-PANoptosis in health and disease.Arch Pharm Res. 2024 Jul;47(7):617-631. doi: 10.1007/s12272-024-01506-0. Epub 2024 Jul 10. Arch Pharm Res. 2024. PMID: 38987410 Review.
-
PANoptosis: a potential new target for programmed cell death in breast cancer treatment and prognosis.Apoptosis. 2024 Apr;29(3-4):277-288. doi: 10.1007/s10495-023-01904-7. Epub 2023 Nov 24. Apoptosis. 2024. PMID: 38001342 Free PMC article. Review.
-
The emerging role of PANoptosis in cancer treatment.Biomed Pharmacother. 2023 Dec;168:115696. doi: 10.1016/j.biopha.2023.115696. Epub 2023 Oct 12. Biomed Pharmacother. 2023. PMID: 37837884 Review.
-
PANoptosis in cancer, the triangle of cell death.Cancer Med. 2023 Dec;12(24):22206-22223. doi: 10.1002/cam4.6803. Epub 2023 Dec 8. Cancer Med. 2023. PMID: 38069556 Free PMC article. Review.
-
From pyroptosis, apoptosis and necroptosis to PANoptosis: A mechanistic compendium of programmed cell death pathways.Comput Struct Biotechnol J. 2021 Aug 3;19:4641-4657. doi: 10.1016/j.csbj.2021.07.038. eCollection 2021. Comput Struct Biotechnol J. 2021. PMID: 34504660 Free PMC article. Review.
Cited by
-
Oxidative stress-mediated PANoptosis and ferroptosis: Exploration of multimodal cell death triggered by an AIE-active nano-photosensitizer via photodynamic therapy.Theranostics. 2025 Jun 9;15(14):6665-6685. doi: 10.7150/thno.111635. eCollection 2025. Theranostics. 2025. PMID: 40585993 Free PMC article.
-
Fucoxanthin from Laminaria japonica Targeting PANoptosis and Ferroptosis Pathways: Insights into Its Therapeutic Potential Against Ovarian Cancer.Mar Drugs. 2025 Mar 12;23(3):123. doi: 10.3390/md23030123. Mar Drugs. 2025. PMID: 40137309 Free PMC article.
-
PANoptosis in Sepsis: A Central Role and Emerging Therapeutic Target.J Inflamm Res. 2025 May 13;18:6245-6261. doi: 10.2147/JIR.S513367. eCollection 2025. J Inflamm Res. 2025. PMID: 40386177 Free PMC article. Review.
-
TRIM56 Modulates YBX1 Degradation to Ameliorate ZBP1-Mediated Neuronal PANoptosis in Spinal Cord Injury.Adv Sci (Weinh). 2024 Nov;11(42):e2407132. doi: 10.1002/advs.202407132. Epub 2024 Sep 18. Adv Sci (Weinh). 2024. PMID: 39291396 Free PMC article.
-
Integrative analysis of immunogenic PANoptosis and experimental validation of cinobufagin-induced activation to enhance glioma immunotherapy.J Exp Clin Cancer Res. 2025 Feb 3;44(1):35. doi: 10.1186/s13046-025-03301-1. J Exp Clin Cancer Res. 2025. PMID: 39901195 Free PMC article.
References
-
- Pan H, Pan J, Li P, Gao J. Characterization of panoptosis patterns predicts survival and immunotherapy response in gastric cancer. Clin Immunol. 2022;238:109019. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

