p32 regulates glycometabolism and TCA cycle to inhibit ccRCC progression via copper-induced DLAT lipoylation oligomerization
- PMID: 38169635
- PMCID: PMC10758103
- DOI: 10.7150/ijbs.84399
p32 regulates glycometabolism and TCA cycle to inhibit ccRCC progression via copper-induced DLAT lipoylation oligomerization
Abstract
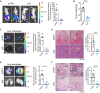
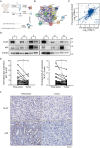
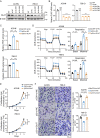
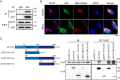
A key player in mitochondrial respiration, p32, often referred to as C1QBP, is mostly found in the mitochondrial matrix. Previously, we showed that p32 interacts with DLAT in the mitochondria. Here, we found that p32 expression was reduced in ccRCC and suppressed progression and metastasis in ccRCC animal models. We observed that increasing p32 expression led to an increase in oxidative phosphorylation by interacting with DLAT, thus, regulating the activation of the pyruvate dehydrogenase complex (PDHc). Mechanistically, reduced p32 expression, in concert with DLAT, suppresses PDHc activity and the TCA cycle. Furthermore, our research discovered that p32 has a direct binding affinity for copper, facilitating the copper-induced oligomerization of lipo-DLAT specifically in ccRCC cells. This finding reveals an innovative function of the p32/DLAT/copper complex in regulating glycometabolism and the TCA cycle in ccRCC. Importantly, our research provides important new understandings of the underlying molecular processes causing the abnormal mitochondrial metabolism linked to this cancer.
Keywords: Clear cell renal cell carcinoma; Copper; DLAT; glycometabolism; p32; tricarboxylic acid cycle.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures


References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A. et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–49. - PubMed
-
- Moch H, Cubilla AL, Humphrey PA, Reuter VE, Ulbright TM. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part A: Renal, Penile, and Testicular Tumours. Eur Urol. 2016;70:93–105. - PubMed
-
- Saeednejad Zanjani L, Madjd Z, Axcrona U, Abolhasani M, Rasti A, Asgari M. et al. Cytoplasmic expression of B7-H3 and membranous EpCAM expression are associated with higher grade and survival outcomes in patients with clear cell renal cell carcinoma. Ann Diagn Pathol. 2020;46:151483. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

