TXNIP in liver sinusoidal endothelial cells ameliorates alcohol-associated liver disease via nitric oxide production
- PMID: 38169654
- PMCID: PMC10758096
- DOI: 10.7150/ijbs.90781
TXNIP in liver sinusoidal endothelial cells ameliorates alcohol-associated liver disease via nitric oxide production
Abstract
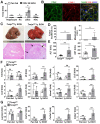
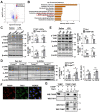
Dysregulation of liver sinusoidal endothelial cell (LSEC) differentiation and function has been reported in alcohol-associated liver disease (ALD). Impaired nitric oxide (NO) production stimulates LSEC capillarization and dysfunction; however, the mechanism underlying NO production remains unclear. Here, we investigated the role of thioredoxin-interacting protein (TXNIP), an important regulator of redox homeostasis, in endothelial cell NO production and its subsequent effects on ALD progression. We found that hepatic TXNIP expression was upregulated in patients with ALD and in ethanol diet-fed mice with high expression in LSECs. Endothelial cell-specific Txnip deficiency (TxnipΔEC) in mice exacerbated alcohol-induced liver injury, inflammation, fibrosis, and hepatocellular carcinoma development. Deletion of Txnip in LSECs led to sinusoidal capillarization, downregulation of NO production, and increased release of proinflammatory cytokines and adhesion molecules, whereas TXNIP overexpression had the opposite effects. Mechanistically, TXNIP interacted with transforming growth factor β-activated kinase 1 (TAK1) and subsequently suppressed the TAK1 pathway. Inhibition of TAK1 activation restored NO production and decreased the levels of proinflammatory cytokines, thereby, blocking liver injury and inflammation in TxnipΔEC mice. Our findings indicate that upregulated TXNIP expression in LSECs serves a protective role in ameliorating ALD. Enhancing TXNIP expression could, therefore, be a potential therapeutic approach for ALD.
Keywords: NO; TAK1; TXNIP; alcohol-associated liver disease; eNOS; liver sinusoidal endothelial cells.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures





References
-
- Hammoutene A, Rautou PE. Role of liver sinusoidal endothelial cells in non-alcoholic fatty liver disease. J Hepatol. 2019;70:1278–91. - PubMed
-
- Poisson J, Lemoinne S, Boulanger C, Durand F, Moreau R, Valla D. et al. Liver sinusoidal endothelial cells: Physiology and role in liver diseases. J Hepatol. 2017;66:212–27. - PubMed
-
- Gracia-Sancho J, Caparros E, Fernandez-Iglesias A, Frances R. Role of liver sinusoidal endothelial cells in liver diseases. Nat Rev Gastroenterol Hepatol. 2021;18:411–31. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

