Glucocorticoid Receptor Antagonism Improves Glucose Metabolism in a Mouse Model of Polycystic Ovary Syndrome
- PMID: 38169733
- PMCID: PMC10758754
- DOI: 10.1210/jendso/bvad162
Glucocorticoid Receptor Antagonism Improves Glucose Metabolism in a Mouse Model of Polycystic Ovary Syndrome
Abstract
Context: Polycystic ovary syndrome (PCOS) is a complex metabolic disorder associated with obesity, insulin resistance, and dyslipidemia. Hyperandrogenism is a major characteristic of PCOS. Increased androgen exposure is believed to deregulate metabolic processes in various tissues as part of the PCOS pathogenesis, predominantly through the androgen receptor (AR). Notably, various metabolic features in PCOS are similar to those observed after excess glucocorticoid exposure.
Objective: We hypothesized that glucocorticoid receptor (GR) signaling is involved in the metabolic symptoms of PCOS.
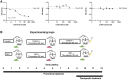
Methods: In a PCOS model of chronic dihydrotestosterone (DHT) exposure in female mice, we investigated whether GR signaling machinery was (de)regulated, and if treatment with a selective GR antagonist alleviated the metabolic symptoms.
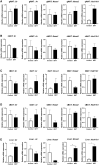
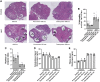
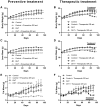
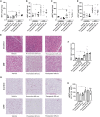
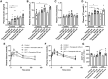
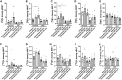
Results: We observed an upregulation of GR messenger RNA expression in the liver after DHT exposure. In white adipose tissues and liver we found that DHT upregulated Hsd11b1, which encodes for the enzyme that converts inactive into active glucocorticoids. We found that preventive but not therapeutic administration of a GR antagonist alleviated DHT-induced hyperglycemia and restored glucose tolerance. We did not observe strong effects of GR antagonism in DHT-exposed mice on other features like total fat mass and lipid accumulation in various tissues.
Conclusion: We conclude that GR activation may play a role in glucose metabolism in DHT-exposed mice.
Keywords: androgen receptor; dihydrotestosterone; glucocorticoid receptor; metabolism; polycystic ovary syndrome.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Endocrine Society.
Figures
References
-
- Meier RK. Polycystic ovary syndrome. Nurs Clin North Am. 2018;53(3):407‐420. - PubMed
-
- Azziz R, Carmina E, Dewailly D, et al. The androgen excess and PCOS society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009;91(2):456‐488. - PubMed
-
- Goodarzi MO, Dumesic DA, Chazenbalk G, Azziz R. Polycystic ovary syndrome: etiology, pathogenesis and diagnosis. Nat Rev Endocrinol. 2011;7(4):219‐231. - PubMed
-
- Pappalardo MA, Russo GT, Pedone A, et al. Very high frequency of the polymorphism for the insulin receptor substrate 1 (IRS-1) at codon 972 (glycine972arginine) in southern Italian women with polycystic ovary syndrome. Horm Metab Res. 2010;42(08):575‐584. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
