SARS-CoV-2 seroprevalence in pregnant women in Kilifi, Kenya from March 2020 to March 2022
- PMID: 38169905
- PMCID: PMC10760635
- DOI: 10.3389/fpubh.2023.1292932
SARS-CoV-2 seroprevalence in pregnant women in Kilifi, Kenya from March 2020 to March 2022
Erratum in
-
Corrigendum: SARS-CoV-2 seroprevalence in pregnant women in Kilifi, Kenya from March 2020 to March 2022.Front Public Health. 2024 Oct 14;12:1500467. doi: 10.3389/fpubh.2024.1500467. eCollection 2024. Front Public Health. 2024. PMID: 39469215 Free PMC article.
Abstract
Background: Seroprevalence studies are an alternative approach to estimating the extent of transmission of SARS-CoV-2 and the evolution of the pandemic in different geographical settings. We aimed to determine the SARS-CoV-2 seroprevalence from March 2020 to March 2022 in a rural and urban setting in Kilifi County, Kenya.
Methods: We obtained representative random samples of stored serum from a pregnancy cohort study for the period March 2020 to March 2022 and tested for antibodies against the spike protein using a qualitative SARS-CoV-2 ELISA kit (Wantai, total antibodies). All positive samples were retested for anti-SARS-CoV-2 anti-nucleocapsid antibodies (Euroimmun, ELISA kits, NCP, qualitative, IgG) and anti-spike protein antibodies (Euroimmun, ELISA kits, QuantiVac; quantitative, IgG).
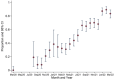
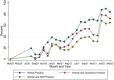
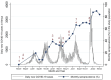
Results: A total of 2,495 (of 4,703 available) samples were tested. There was an overall trend of increasing seropositivity from a low of 0% [95% CI 0-0.06] in March 2020 to a high of 89.4% [95% CI 83.36-93.82] in Feb 2022. Of the Wantai test-positive samples, 59.7% [95% CI 57.06-62.34] tested positive by the Euroimmun anti-SARS-CoV-2 NCP test and 37.4% [95% CI 34.83-40.04] tested positive by the Euroimmun anti-SARS-CoV-2 QuantiVac test. No differences were observed between the urban and rural hospital but villages adjacent to the major highway traversing the study area had a higher seroprevalence.
Conclusion: Anti-SARS-CoV-2 seroprevalence rose rapidly, with most of the population exposed to SARS-CoV-2 within 23 months of the first cases. The high cumulative seroprevalence suggests greater population exposure to SARS-CoV-2 than that reported from surveillance data.
Keywords: COVID-19; Kenya; SARS-CoV-2; antibodies; pregnancy; seroprevalence.
Copyright © 2023 Koech, Omuse, Mugo, Mwaniki, Mutunga, Mukhanya, Wanje, Mwashigadi, Katana, Craik, von Dadelszen, Le Doare, Temmerman, periCOVID-Africa and The PRECISE Network.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Amahazion F. Exploring national COVID-19 variability across sub-Saharan Africa. J Glob Health Rep. (2021) 5:5. doi: 10.29392/001c.24941 - DOI
-
- World Health Organization . Global surveillance for COVID-19 caused by human infection with COVID-19 virus: Interim guidance, 20 March 2020: World Health Organization (2020) Available at: https://apps.who.int/iris/handle/10665/331506.
-
- Kumar MS, Bhatnagar T, Manickam P, Kumar VS, Rade K, Shah N, et al. . National sero-surveillance to monitor the trend of SARS-CoV-2 infection transmission in India: protocol for community-based surveillance. Indian J Med Res. (2020) 151:419–23. doi: 10.4103/ijmr.IJMR_1818_20, PMID: - DOI - PMC - PubMed
-
- World Health Organisation and Joint United Nations Programme on HIV/AIDS . Reconciling antenatal clinic-based surveillance and population-based survey estimates of HIV prevalence in sub-saharan Africa. (2003). Available at: http://data.unaids.org/una-docs/anc-population_surveys_report_en.pdf.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

