Short-term effectiveness of single-dose intranasal spray COVID-19 vaccine against symptomatic SARS-CoV-2 Omicron infection in healthcare workers: a prospective cohort study
- PMID: 38169940
- PMCID: PMC10758709
- DOI: 10.1016/j.eclinm.2023.102374
Short-term effectiveness of single-dose intranasal spray COVID-19 vaccine against symptomatic SARS-CoV-2 Omicron infection in healthcare workers: a prospective cohort study
Abstract
Background: The pivotal phase 3 efficacy clinical trial has demonstrated that a two-dose regimen of dNS1-RBD (Beijing Wantai Biological Pharmacy Enterprise, Beijing, China) is well-tolerated and provides wide protection against SARS-CoV-2 infection. However, the effectiveness of a single-dose regimen is still unknown. We aimed to estimate the effectiveness of one-dose of dNS1-RBD against symptomatic Omicron infections in real-world conditions.
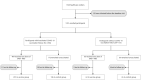
Methods: This prospective cohort study was conducted during an Omicron outbreak among healthcare workers in Xiamen, China, from December 22, 2022 to January 16, 2023. Participants chose to receive single-dose of dNS1-RBD or remain unvaccinated based on personal preference. Healthcare workers daily validated their SARS-CoV-2 infection status, using either RT-PCR or rapid antigen test. A survey questionnaire was conducted to gather information on acute symptoms from individuals infected with SARS-CoV-2. The primary outcome was the symptomatic SARS-CoV-2 infections after enrollment in the dNS1-RBD recipients or the control group among all participants and by prior COVID-19 vaccination status.
Findings: On December 22, 2022, a total of 1391 eligible participants without a history of prior SARS-CoV-2 infection were enrolled. Among them, 550 received single-dose of dNS1-RBD, while 841 remained unvaccinated. In the total cohort, the range of follow-up time was 1∼26 days. During the study period, a total of 880 symptomatic SARS-CoV-2 infections were identified in the total cohort. The adjusted vaccine effectiveness against symptomatic SARS-CoV-2 infections and the infections requiring medical attention were 19.0% (95% CI: 6.7, 29.7, P = 0.004) and 59.4% (95% CI: 25.1, 78.0, P = 0.004) in the total cohort, 11.6% (95% CI: -2.4, 23.7, P = 0.100) and 55.3% (95% CI: 15.3, 76.4, P = 0.014) in the participants with inactivated COVID-19 vaccination history, as well as 87.0% (95% CI: 72.6, 93.9, P < 0.001) and 84.2% (95% CI: -41.8, 98.2, P = 0.099) in the naïve participants, respectively.
Interpretation: When administered as a booster to individuals with a history of inactivated COVID-19 vaccination, a single-dose of dNS1-RBD provides protection against infections requiring medical attention at least in the short-term after vaccination. The data also showed that a single-dose of dNS1-RBD is protective against symptomatic SARS-CoV-2 infections as a primary immunization for individuals without prior exposure, but due to the limited sample size of naïve participants, further research with a larger sample size is needed to make a solid conclusion.
Funding: Xiamen Science and Technology Bureau 2022 General Science and Technology Plan Project and the Bill & Melinda Gates Foundation.
Keywords: Effectiveness; Intranasal spray COVID-19 vaccine; SARS-CoV-2; Single-dose regimen; dNS1-RBD.
© 2023 The Author(s).
Conflict of interest statement
All authors declare no competing interests.
Figures

Similar articles
-
Safety and efficacy of the intranasal spray SARS-CoV-2 vaccine dNS1-RBD: a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial.Lancet Respir Med. 2023 Dec;11(12):1075-1088. doi: 10.1016/S2213-2600(23)00349-1. Epub 2023 Nov 15. Lancet Respir Med. 2023. PMID: 37979588 Free PMC article. Clinical Trial.
-
Safety and immunogenicity of a live-attenuated influenza virus vector-based intranasal SARS-CoV-2 vaccine in adults: randomised, double-blind, placebo-controlled, phase 1 and 2 trials.Lancet Respir Med. 2022 Aug;10(8):749-760. doi: 10.1016/S2213-2600(22)00131-X. Epub 2022 May 26. Lancet Respir Med. 2022. PMID: 35644168 Free PMC article. Clinical Trial.
-
Effectiveness of BNT162b2 and CoronaVac COVID-19 vaccination against asymptomatic and symptomatic infection of SARS-CoV-2 omicron BA.2 in Hong Kong: a prospective cohort study.Lancet Infect Dis. 2023 Apr;23(4):421-434. doi: 10.1016/S1473-3099(22)00732-0. Epub 2022 Dec 12. Lancet Infect Dis. 2023. PMID: 36521506 Free PMC article.
-
Facing the Omicron variant-how well do vaccines protect against mild and severe COVID-19? Third interim analysis of a living systematic review.Front Immunol. 2022 Aug 24;13:940562. doi: 10.3389/fimmu.2022.940562. eCollection 2022. Front Immunol. 2022. PMID: 36091023 Free PMC article.
-
Universal screening for SARS-CoV-2 infection: a rapid review.Cochrane Database Syst Rev. 2020 Sep 15;9(9):CD013718. doi: 10.1002/14651858.CD013718. Cochrane Database Syst Rev. 2020. PMID: 33502003 Free PMC article.
Cited by
-
Reduced SARS-CoV-2 Infection Rates in Lab Workers Conducting Nucleic Acid Testing: Controlling for the Healthy Worker Effect.J Epidemiol Glob Health. 2025 Jan 29;15(1):10. doi: 10.1007/s44197-025-00343-8. J Epidemiol Glob Health. 2025. PMID: 39878891 Free PMC article.
-
Intranasal influenza-vectored COVID-19 vaccines confer broad protection against SARS-CoV-2 XBB variants in hamsters.PNAS Nexus. 2024 May 3;3(5):pgae183. doi: 10.1093/pnasnexus/pgae183. eCollection 2024 May. PNAS Nexus. 2024. PMID: 38800610 Free PMC article.
-
A randomized phase I trial of intranasal SARS-CoV-2 vaccine dNS1-RBD in children aged 3-17 years.NPJ Vaccines. 2025 Mar 17;10(1):50. doi: 10.1038/s41541-025-01096-y. NPJ Vaccines. 2025. PMID: 40097460 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

