Efficacy and safety of onasemnogene abeparvovec in children with spinal muscular atrophy type 1: real-world evidence from 6 infusion centres in the United Kingdom
- PMID: 38169987
- PMCID: PMC10758961
- DOI: 10.1016/j.lanepe.2023.100817
Efficacy and safety of onasemnogene abeparvovec in children with spinal muscular atrophy type 1: real-world evidence from 6 infusion centres in the United Kingdom
Abstract
Background: Real-world data on the efficacy and safety of onasemnogene abeparvovec (OA) in spinal muscular atrophy (SMA) are needed, especially to overcome uncertainties around its use in older and heavier children. This study evaluated the efficacy and safety of OA in patients with SMA type 1 in the UK, including patients ≥2 years old and weighing ≥13.5 kg.
Methods: This observational cohort study used data from patients with genetically confirmed SMA type 1 treated with OA between May 2021 and January 2023, at 6 infusion centres in the United Kingdom. Functional outcomes were assessed using age-appropriate functional scales. Safety analyses included review of liver function, platelet count, cardiac assessments, and steroid requirements.
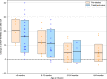
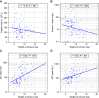
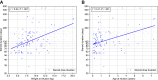
Findings: Ninety-nine patients (45 SMA therapy-naïve) were treated with OA (median age at infusion: 10 [range, 0.6-89] months; median weight: 7.86 [range, 3.2-20.2] kg; duration of follow-up: 3-22 months). After OA infusion, mean ± SD change in CHOP-INTEND score was 11.0 ± 10.3 with increased score in 66/78 patients (84.6%); patients aged <6 months had a 13.9 points higher gain in CHOP-INTEND score than patients ≥2 years (95% CI, 6.8-21.0; P < 0.001). Asymptomatic thrombocytopenia (71/99 patients; 71.7%), asymptomatic troponin-I elevation (30/89 patients; 33.7%) and transaminitis (87/99 patients; 87.9%) were reported. No thrombotic microangiopathy was observed. Median steroid treatment duration was 97 (range, 28-548) days with dose doubled in 35/99 patients (35.4%). There were 22.5-fold increased odds of having a transaminase peak >100 U/L (95% CI, 2.3-223.7; P = 0.008) and 21.2-fold increased odds of steroid doubling, as per treatment protocol (95% CI, 2.2-209.2; P = 0.009) in patients weighing ≥13.5 kg versus <8.5 kg. Weight at infusion was positively correlated with steroid treatment duration (r = 0.43; P < 0.001). Worsening transaminitis, despite doubling of oral prednisolone, led to treatment with intravenous methylprednisolone in 5 children. Steroid-sparing immunosuppressants were used in 5 children to enable steroid weaning. Two deaths apparently unrelated to OA were reported.
Interpretation: OA led to functional improvements and was well tolerated with no persistent clinical complications, including in older and heavier patients.
Funding: Novartis Innovative Therapies AG provided a grant for independent medical writing services.
Keywords: Efficacy; Follow-up; Gene therapy; Longitudinal; Motor neuron disorder; Onasemnogene abeparvovec; Real-world experience; SMA; Safety; Spinal muscular atrophy; United Kingdom; Zolgensma.
© 2023 The Author(s).
Conflict of interest statement
Vasantha Gowda has participated in scientific advisory boards, scientific symposia and teaching initiatives for Biogen, Roche, Novartis, Wave Life Sciences, PTC therapeutics and Pfizer, received honoraria from Neurology Academy, Roche, Novartis and has been involved as principal investigator with PTC therapeutics, Wave Life Sciences and Catabasis. Mark Atherton has received conference sponsorship from Novartis. Jennie Sheehan has participated in advisory boards for Roche, received conference sponsorships from Roche and Biogen and honoraria from Novartis. Mariacristina Scoto has been involved as principal investigator in clinical trials from Roche, Biogen and Novartis and has participated in Scientific Advisory boards and teaching initiatives for Roche, Novartis and Biogen. Giovanni Baranello has been involved as principal investigator of clinical trials sponsored by Roche, Novartis, Sarepta, Pfizer, NS Pharma, Reveragen, Scholar Rock, and has received speaker and/or consulting fees from Sarepta, PTC Therapeutics, Pfizer, Biogen, Novartis Gene Therapies, Inc. (AveXis), and Roche, and grants from Sarepta, Roche and Novartis Gene Therapies. Archana Murugan has received conference sponsorship from Roche. Anil Dhawan has participated in advisory boards for Novartis, BitBio, Aspect Bio, Astellas. Michael Eyre is supported by Action Medical Research and the British Paediatric Neurology Association. Laurent Servais reports participation in advisory boards and scientific symposia from Biogen, Roche, Novartis, Scholar Rock and BioHaven and grants from the aforementioned and Zentech, Perkin Almers. Francesco Muntoni reports participation in advisory boards and scientific symposia from Biogen, Roche and Novartis and funding from Biogen and Roche for the SMA REACH National database. Min Ong has participated in advisory boards for Biogen, Novartis, Roche & CSL Behring and has received conference sponsorship from Roche and speaker fees from Biogen, and honorarium from Neurology Academy. Imelda Hughes has received honoraria from Santhera, Roche, PTC Therapeutics, Sarepta, Biogen and Novartis, conference sponsorships from PTC Therapeutics, Novartis and Biogen. Heinz Jungbluth has participated in advisory board activities for Pfizer, Astellas, Audentes and Armgo. Elizabeth Wraige has undertaken consultancy work for Novartis. Maria Vanegas has received conference sponsorship from Novartis and meeting sponsorship from SMA Europe. Sandya Tirupathi has received conference sponsorships from Novartis and PTC. Marjorie Illingworth has received conference sponsorships from Roche and Biogen. Adnan Manzur received honorarium and meeting sponsorship from Pi Healthcare. Sarah D’Urso has received conference sponsorship from Biogen. Tracey Willis has received honorarium and conference sponsorship from Novartis. Sinead Warner has received conference sponsorship from Novartis and consultancy fees from Roche. No other disclosures were reported.
Figures
References
-
- Lunn M.R., Wang C.H. Spinal muscular atrophy. Lancet. 2008;371(9630):2120–2133. - PubMed
-
- Prior T.W., Leach M.E., Finanger E. GeneReviews®; 1993. Spinal muscular atrophy. - PubMed
-
- Coovert D.D., Le T.T., McAndrew P.E., et al. The survival motor neuron protein in spinal muscular atrophy. Hum Mol Genet. 1997;6(8):1205–1214. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

