Long-term outcomes with haloperidol versus placebo in acutely admitted adult ICU patients with delirium
- PMID: 38170227
- PMCID: PMC10811094
- DOI: 10.1007/s00134-023-07282-7
Long-term outcomes with haloperidol versus placebo in acutely admitted adult ICU patients with delirium
Abstract
Purpose: We assessed long-term outcomes in acutely admitted adult patients with delirium treated in intensive care unit (ICU) with haloperidol versus placebo.
Methods: We conducted pre-planned analyses of 1-year outcomes in the Agents Intervening against Delirium in the ICU (AID-ICU) trial, including mortality and health-related quality of life (HRQoL) assessed by Euroqol (EQ) 5-dimension 5-level questionnaire (EQ-5D-5L) index values and EQ visual analogue scale (EQ VAS) (deceased patients were assigned the numeric value zero). Outcomes were analysed using logistic and linear regressions with bootstrapping and G-computation, all with adjustment for the stratification variables (site and delirium motor subtype) and multiple imputations for missing HRQoL values.
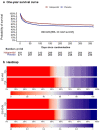
Results: At 1-year follow-up, we obtained vital status for 96.2% and HRQoL data for 83.3% of the 1000 randomised patients. One-year mortality was 224/501 (44.7%) in the haloperidol group versus 251/486 (51.6%) in the placebo group, with an adjusted absolute risk difference of - 6.4%-points (95% confidence interval [CI] - 12.8%-points to - 0.2%-points; P = 0.045). These results were largely consistent across the secondary analyses. For HRQoL, the adjusted mean differences were 0.04 (95% CI - 0.03 to 0.11; P = 0.091) for EQ-5D-5L-5L index values, and 3.3 (95% CI - 9.3 to 17.5; P = 0.142) for EQ VAS.
Conclusions: In acutely admitted adult ICU patients with delirium, haloperidol treatment reduced mortality at 1-year follow-up, but did not statistically significantly improve HRQoL.
Trial registration: ClinicalTrials.gov NCT03392376.
Keywords: Delirium; Health-related quality of life; ICU; Long-term outcomes; Mortality; Treatment.
© 2023. The Author(s).
Conflict of interest statement
AG, MBNK, MOC and AP are affiliated with the Department of Intensive Care at Rigshospitalet, which has received funding for other projects from The Novo Nordisk Foundation, Pfizer, and Fresenius Kabi, Sygeforsikringen “danmark”. AP has received an honorarium from Novartis for the participation in an advisory board. AP is on DSMB for The Mega-Rox trial, UK-Rox trial, Bone Zone trial. BSR are affiliated with the Department of Anaesthesia and Intensive Care, Aalborg University Hospital, Aalborg, Denmark, which has received funding for other project from Novo Nordisk Foundation, Danish Ministry of Education and Science and Beckett Foundation. MHB are affiliated with the Department of Anaesthesia and Intensive Care at Copenhagen University Hospital – North Zealand, which has received funding for other research projects from The Novo Nordisk Foundation, Sygeforsikringen “danmark”, Toyota Foundation, A.P. Moeller Foundation, Frimodt-Heineke Foundation, Svend Andersen Foundation, Ehrenreich Foundation, and Olga Bryde Nielsen Foundation, and has conducted contract research for AM-Pharma (the REVIVAL trial) and Inotrem (ASTONISH trial). MHB has received an honorarium from AM-Pharma for participation in an advisory board. TL is a member of DSMB for Novo Nordisk and Leo Pharma study. BE is part of the Advisory Board of Eli Lilly Denmark A/S, Janssen-Cilag, Lundbeck Pharma A/S, and Takeda Pharmaceutical Company Ltd; and has received lecture fees from Bristol-Myers Squibb, Boehringer Ingelheim, Otsuka Pharma Scandinavia AB, Eli Lilly Company, and Lundbeck Pharma A/S. JH has received funding from Paulo foundation, Finska Läkaresällskapet, NordForsk and Tor och Kirsti Johanssons Hjärt och Cancerstiftelse. GC has received istituational research grants from Neuroptics and Integra and is a board member at Neuroptics, Integra, Biogen, Invex Therapeutics and Idorsia. All other authors have no conflicts to disclose.
Figures


References
-
- Association AP. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®) American Psychiatric Pub; 2013. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

