Acute antiarrhythmic effects of SGLT2 inhibitors-dapagliflozin lowers the excitability of atrial cardiomyocytes
- PMID: 38170280
- PMCID: PMC10837223
- DOI: 10.1007/s00395-023-01022-0
Acute antiarrhythmic effects of SGLT2 inhibitors-dapagliflozin lowers the excitability of atrial cardiomyocytes
Abstract
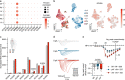
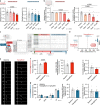
In recent years, SGLT2 inhibitors have become an integral part of heart failure therapy, and several mechanisms contributing to cardiorenal protection have been identified. In this study, we place special emphasis on the atria and investigate acute electrophysiological effects of dapagliflozin to assess the antiarrhythmic potential of SGLT2 inhibitors. Direct electrophysiological effects of dapagliflozin were investigated in patch clamp experiments on isolated atrial cardiomyocytes. Acute treatment with elevated-dose dapagliflozin caused a significant reduction of the action potential inducibility, the amplitude and maximum upstroke velocity. The inhibitory effects were reproduced in human induced pluripotent stem cell-derived cardiomyocytes, and were more pronounced in atrial compared to ventricular cells. Hypothesizing that dapagliflozin directly affects the depolarization phase of atrial action potentials, we examined fast inward sodium currents in human atrial cardiomyocytes and found a significant decrease of peak sodium current densities by dapagliflozin, accompanied by a moderate inhibition of the transient outward potassium current. Translating these findings into a porcine large animal model, acute elevated-dose dapagliflozin treatment caused an atrial-dominant reduction of myocardial conduction velocity in vivo. This could be utilized for both, acute cardioversion of paroxysmal atrial fibrillation episodes and rhythm control of persistent atrial fibrillation. In this study, we show that dapagliflozin alters the excitability of atrial cardiomyocytes by direct inhibition of peak sodium currents. In vivo, dapagliflozin exerts antiarrhythmic effects, revealing a potential new additional role of SGLT2 inhibitors in the treatment of atrial arrhythmias.
Keywords: Atrial action potential; Atrial fibrillation; Dapagliflozin; NaV1.5; SGLT2 inhibitors.
© 2024. The Author(s).
Conflict of interest statement
C.S. received research funding from Böhringer Ingelheim, Ingelheim, Germany. All other authors declare that they have no competing interests.
Figures





References
-
- Bode D, Semmler L, Wakula P, Hegemann N, Primessnig U, Beindorff N, Powell D, Dahmen R, Ruetten H, Oeing C, Alogna A, Messroghli D, Pieske BM, Heinzel FR, Hohendanner F. Dual SGLT-1 and SGLT-2 inhibition improves left atrial dysfunction in HFpEF. Cardiovasc Diabetol. 2021;20:7. doi: 10.1186/s12933-020-01208-z. - DOI - PMC - PubMed
-
- Böhm M, Slawik J, Brueckmann M, Mattheus M, George JT, Ofstad AP, Inzucchi SE, Fitchett D, Anker SD, Marx N, Wanner C, Zinman B, Verma S. Efficacy of empagliflozin on heart failure and renal outcomes in patients with atrial fibrillation: data from the EMPA-REG OUTCOME trial. Eur J Heart Fail. 2020;22:126–135. doi: 10.1002/ejhf.1663. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

