Gut metabolite L-lactate supports Campylobacter jejuni population expansion during acute infection
- PMID: 38170751
- PMCID: PMC10786315
- DOI: 10.1073/pnas.2316540120
Gut metabolite L-lactate supports Campylobacter jejuni population expansion during acute infection
Abstract
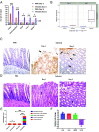
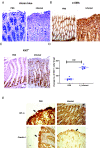
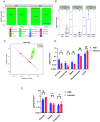
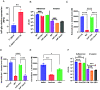
How the microaerobic pathogen Campylobacter jejuni establishes its niche and expands in the gut lumen during infection is poorly understood. Using 6-wk-old ferrets as a natural disease model, we examined this aspect of C. jejuni pathogenicity. Unlike mice, which require significant genetic or physiological manipulation to become colonized with C. jejuni, ferrets are readily infected without the need to disarm the immune system or alter the gut microbiota. Disease after C. jejuni infection in ferrets reflects closely how human C. jejuni infection proceeds. Rapid growth of C. jejuni and associated intestinal inflammation was observed within 2 to 3 d of infection. We observed pathophysiological changes that were noted by cryptic hyperplasia through the induction of tissue repair systems, accumulation of undifferentiated amplifying cells on the colon surface, and instability of HIF-1α in colonocytes, which indicated increased epithelial oxygenation. Metabolomic analysis demonstrated that lactate levels in colon content were elevated in infected animals. A C. jejuni mutant lacking lctP, which encodes an L-lactate transporter, was significantly decreased for colonization during infection. Lactate also influences adhesion and invasion by C. jejuni to a colon carcinoma cell line (HCT116). The oxygenation required for expression of lactate transporter (lctP) led to identification of a putative thiol-based redox switch regulator (LctR) that may repress lctP transcription under anaerobic conditions. Our work provides better insights into the pathogenicity of C. jejuni.
Keywords: Campylobacter jejuni; ferret model; inflammation; lactate.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures

Update of
-
Gut metabolite L-lactate supports Campylobacter jejuni population expansion during acute infection.bioRxiv [Preprint]. 2023 Oct 2:2023.10.02.560557. doi: 10.1101/2023.10.02.560557. bioRxiv. 2023. Update in: Proc Natl Acad Sci U S A. 2024 Jan 9;121(2):e2316540120. doi: 10.1073/pnas.2316540120. PMID: 37873437 Free PMC article. Updated. Preprint.
References
-
- Tacconelli E., et al. , Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 18, 318–327 (2018). - PubMed
-
- Manfredi R., Nanetti A., Ferri M., Chiodo F., Fatal Campylobacter jejuni bacteraemia in patients with AIDS. J. Med. Microbiol. 48, 601–603 (1999). - PubMed
-
- Fitzgerald C., Campylobacter. Clin. Lab. Med. 35, 289–298 (2015). - PubMed
-
- Young K. T., Davis L. M., Dirita V. J., Campylobacter jejuni: Molecular biology and pathogenesis. Nat. Rev. Microbiol. 5, 665–679 (2007). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

