Characterizing Acute-Onset Small Fiber Neuropathy
- PMID: 38170952
- PMCID: PMC10766082
- DOI: 10.1212/NXI.0000000000200195
Characterizing Acute-Onset Small Fiber Neuropathy
Abstract
Background and objectives: Immune-mediated small fiber neuropathy (SFN) is increasingly recognized. Acute-onset SFN (AOSFN) remains poorly described. Herein, we report a series of AOSFN cases in which immune origins are debatable.
Methods: We included consecutive patients with probable or definite AOSFN. Diagnosis of SFN was based on the NEURODIAB criteria. Acute onset was considered when the maximum intensity and extension of both symptoms and signs were reached within 28 days. We performed the following investigations: clinical examination, neurophysiologic assessment encompassing a nerve conduction study to rule out large fiber neuropathy, laser-evoked potentials (LEPs), warm detection thresholds (WDTs), electrochemical skin conductance (ESC), epidermal nerve fiber density (ENF), and patient serum reactivity against mouse sciatic nerve teased fibers, mouse dorsal root ganglion (DRG) sections, and cultured DRG. The serum reactivity of healthy subjects (n = 10) and diseased controls (n = 12) was also analyzed. Data on baseline characteristics, biological investigations, and disease course were collected.
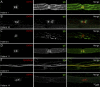
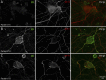
Results: Twenty patients presenting AOSFN were identified (60% women; median age: 44.2 years [interquartile range: 35.7-56.2]). SFN was definite in 18 patients (90%) and probable in 2 patients. A precipitating event was present in 16 patients (80%). The median duration of the progression phase was 14 days [5-28]. Pain was present in 17 patients (85%). Twelve patients (60%) reported autonomic involvement. The clinical pattern was predominantly non-length-dependent (85%). Diagnosis was confirmed by abnormal LEPs (60%), ENF (55%), WDT (39%), or ESC (31%). CSF analysis was normal in 5 of 5 patients. Antifibroblast growth factor 3 antibodies were positive in 4 of 18 patients (22%) and anticontactin-associated protein-2 antibodies in one patient. In vitro studies showed IgG immunoreactivity against nerve tissue in 14 patients (70%), but not in healthy subjects or diseased controls. Patient serum antibodies bound to unmyelinated fibers, Schwann cells, juxtaparanodes, paranodes, or DRG. Patients' condition improved after a short course of oral corticosteroids (3/3). Thirteen patients (65%) showed partial or complete recovery. Others displayed relapses or a chronic course.
Discussion: AOSFN primarily presents as an acute, non-length-dependent, symmetric painful neuropathy with a variable disease course. An immune-mediated origin has been suggested based on in vitro immunohistochemical studies.
Conflict of interest statement
T. Gendre received consulting fees from Alnylam, symposium fees from ArgenX, and supports for congress from Alnylam, Pfizer, LFB, CSL Behring, and Elivie. J.P. Lefaucheur reports no conflict of interest. T. Nordine reports no conflict of interest. Y. Baba-Amer reports no conflict of interest. F.J. Authier reports no conflict of interest. J. Devaux received a research grant form CSL Behring and is conducting a research contract with ArgenX. A. Créange received grants and nonfinancial support from Medday, personal fees from Merck, grants and personal fees from Alexion, Biogen, Novartis, and Roche outside the submitted work. Go to
Figures

Similar articles
-
Diagnosis of small fiber neuropathy: A comparative study of five neurophysiological tests.Neurophysiol Clin. 2015 Dec;45(6):445-55. doi: 10.1016/j.neucli.2015.09.012. Epub 2015 Nov 17. Neurophysiol Clin. 2015. PMID: 26596193
-
The role of skin biopsy in differentiating small-fiber neuropathy from ganglionopathy.Eur J Neurol. 2018 Jun;25(6):848-853. doi: 10.1111/ene.13608. Epub 2018 Apr 6. Eur J Neurol. 2018. PMID: 29493845
-
The diagnostic criteria for small fibre neuropathy: from symptoms to neuropathology.Brain. 2008 Jul;131(Pt 7):1912-25. doi: 10.1093/brain/awn093. Epub 2008 Jun 4. Brain. 2008. PMID: 18524793 Free PMC article.
-
Small fiber neuropathy.Acta Neurol Scand. 2022 May;145(5):493-503. doi: 10.1111/ane.13591. Epub 2022 Feb 7. Acta Neurol Scand. 2022. PMID: 35130356 Review.
-
Non-length-dependent small fiber neuropathy: Not a matter of stockings and gloves.Muscle Nerve. 2022 Jan;65(1):10-28. doi: 10.1002/mus.27379. Epub 2021 Aug 9. Muscle Nerve. 2022. PMID: 34374103 Review.
Cited by
-
Increasing associations of long-COVID with small-fiber neuropathy.Pain. 2024 Sep 1;165(9):e93-e95. doi: 10.1097/j.pain.0000000000003260. Epub 2024 May 3. Pain. 2024. PMID: 39159474 No abstract available.
-
Post-COVID Small Fiber Neuropathy, Implications of Innate Immunity, and Challenges on IVIG Therapy.Neurol Neuroimmunol Neuroinflamm. 2024 May;11(3):e200248. doi: 10.1212/NXI.0000000000200248. Epub 2024 Apr 17. Neurol Neuroimmunol Neuroinflamm. 2024. PMID: 38630951 Free PMC article. No abstract available.
-
Relapsing-Remitting Immunotherapy Responsive Small-Fiber Neuropathy: Longitudinal Tracking Through 10 Years Including Pregnancies.Neurol Neuroimmunol Neuroinflamm. 2024 Sep;11(5):e200286. doi: 10.1212/NXI.0000000000200286. Epub 2024 Jul 24. Neurol Neuroimmunol Neuroinflamm. 2024. PMID: 39047208 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
