Enantioselective Aziridination of Unactivated Terminal Alkenes Using a Planar Chiral Rh(III) Indenyl Catalyst
- PMID: 38170978
- PMCID: PMC10797617
- DOI: 10.1021/jacs.3c10637
Enantioselective Aziridination of Unactivated Terminal Alkenes Using a Planar Chiral Rh(III) Indenyl Catalyst
Abstract
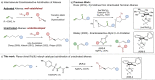
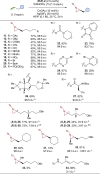
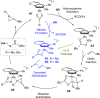
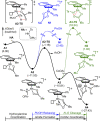
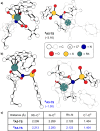
Chiral aziridines are important structural motifs found in natural products and various target molecules. They serve as versatile building blocks for the synthesis of chiral amines. While advances in catalyst design have enabled robust methods for enantioselective aziridination of activated olefins, simple and abundant alkyl-substituted olefins pose a significant challenge. In this work, we introduce a novel approach utilizing a planar chiral rhodium indenyl catalyst to facilitate the enantioselective aziridination of unactivated alkenes. This transformation exhibits a remarkable degree of functional group tolerance and displays excellent chemoselectivity favoring unactivated alkenes over their activated counterparts, delivering a wide range of enantioenriched high-value chiral aziridines. Computational studies unveil a stepwise aziridination mechanism in which alkene migratory insertion plays a central role. This process results in the formation of a strained four-membered metallacycle and serves as both the enantio- and rate-determining steps in the overall reaction.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Lu P. Recent developments in regioselective ring opening of aziridines. Tetrahedron 2010, 66, 2549–2560. 10.1016/j.tet.2010.01.077. - DOI
-
- Vervisch K.; D’Hooghe M.; Tornroos K. W.; De Kimpe N. Synthesis of stereodefined piperidines from aziridines and their transformation into conformationally constrained amino acids, amino alcohols and 2,7-diazabicyclo[3.3.1]nonanes. J. Org. Chem. 2010, 75, 7734–7744. 10.1021/jo101646u. - DOI - PubMed
-
- Hu X. E. Nucleophilic ring opening of aziridines. Tetrahedron 2004, 60, 2701–2743. 10.1016/j.tet.2004.01.042. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

