Mesenchymal Stem Cells Combined With Electroacupuncture Treatment Regulate the Subpopulation of Macrophages and Astrocytes to Facilitate Axonal Regeneration in Transected Spinal Cord
- PMID: 38171303
- PMCID: PMC10762392
- DOI: 10.14245/ns.2346824.412
Mesenchymal Stem Cells Combined With Electroacupuncture Treatment Regulate the Subpopulation of Macrophages and Astrocytes to Facilitate Axonal Regeneration in Transected Spinal Cord
Abstract
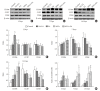
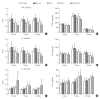
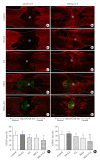
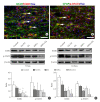
Objective: Herein, we investigated whether mesenchymal stem cells (MSCs) transplantation combined with electroacupuncture (EA) treatment could decrease the proportion of proinflammatory microglia/macrophages and neurotoxic A1 reactive astrocytes and inhibit glial scar formation to enhance axonal regeneration after spinal cord injury (SCI).
Methods: Adult rats were divided into 5 groups after complete transection of the spinal cord at the T10 level: a control group, a nonacupoint EA (NA-EA) group, an EA group, an MSC group, and an MSCs+EA group. Immunofluorescence labeling, quantitative real-time polymerase chain reaction, enzyme-linked immunosorbent assay, and Western blots were performed.
Results: The results showed that MSCs+EA treatment reduced the proportion of proinflammatory M1 subtype microglia/macrophages, but increased the differentiation of anti-inflammatory M2 phenotype cells, thereby suppressing the mRNA and protein expression of proinflammatory cytokines (tumor necrosis factor-α and IL-1β) and increasing the expression of an anti-inflammatory cytokine (interleukin [IL]-10) on days 7 and 14 after SCI. The changes in expression correlated with the attenuated neurotoxic A1 reactive astrocytes and glial scar, which in turn facilitated the axonal regeneration of the injured spinal cord. In vitro, the proinflammatory cytokines increased the level of proliferation of astrocytes and increased the expression levels of C3, glial fibrillary acidic protein, and chondroitin sulfate proteoglycan. These effects were blocked by administering inhibitors of ErbB1 and signal transducer and activator of transcription 3 (STAT3) (AG1478 and AG490) and IL-10.
Conclusion: These findings showed that MSCs+EA treatment synergistically regulated the microglia/macrophage subpopulation to reduce inflammation, the formation of neurotoxic A1 astrocytes, and glial scars. This was achieved by downregulating the ErbB1-STAT3 signal pathway, thereby providing a favorable microenvironment conducive to axonal regeneration after SCI.
Keywords: Axonal regeneration; Electroacupuncture; Macrophages; Mesenchymal stem cells; Microglia; Reactive astrocytes; Spinal cord injury.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Electroacupuncture stimulation inhibited astrogliosis and microglia polarisation to alleviate spinal cord injury via Janus kinase 2/signal transducer and activator of transcription 3 signalling pathway.Folia Histochem Cytobiol. 2025;63(1):28-40. doi: 10.5603/fhc.104273. Folia Histochem Cytobiol. 2025. PMID: 40421823
-
Bone marrow mesenchymal stem cells and electroacupuncture downregulate the inhibitor molecules and promote the axonal regeneration in the transected spinal cord of rats.Cell Transplant. 2011;20(4):475-91. doi: 10.3727/096368910X528102. Epub 2010 Sep 30. Cell Transplant. 2011. PMID: 20887664
-
Electroacupuncture promotes the differentiation of transplanted bone marrow mesenchymal stem cells overexpressing TrkC into neuron-like cells in transected spinal cord of rats.Cell Transplant. 2013;22(1):65-86. doi: 10.3727/096368912X655037. Epub 2012 Sep 21. Cell Transplant. 2013. PMID: 23006476
-
Biomaterial-supported MSC transplantation enhances cell-cell communication for spinal cord injury.Stem Cell Res Ther. 2021 Jan 7;12(1):36. doi: 10.1186/s13287-020-02090-y. Stem Cell Res Ther. 2021. PMID: 33413653 Free PMC article. Review.
-
Neuroinflammation and Scarring After Spinal Cord Injury: Therapeutic Roles of MSCs on Inflammation and Glial Scar.Front Immunol. 2021 Dec 2;12:751021. doi: 10.3389/fimmu.2021.751021. eCollection 2021. Front Immunol. 2021. PMID: 34925326 Free PMC article. Review.
Cited by
-
Therapeutic Transplantation of Human Central Nervous System Organoids for Neural Reconstruction.Int J Mol Sci. 2024 Aug 5;25(15):8540. doi: 10.3390/ijms25158540. Int J Mol Sci. 2024. PMID: 39126108 Free PMC article. Review.
References
-
- Karimi-Abdolrezaee S, Billakanti R. Reactive astrogliosis after spinal cord injury-beneficial and detrimental effects. Mol Neurobiol. 2012;46:251–64. - PubMed
-
- Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–64. - PubMed
-
- Martinez FO, Sica A, Mantovani A, et al. Macrophage activation and polarization. Front Biosci. 2008;13:453–61. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

